Method for synthesizing cis-3-tetradecanoacetate and trans-3-tetradecanoacetate
A technology of tetradecenyl acetate and tetradecenol, which is applied in the field of insect attractants, can solve the problems of high price, dangerous synthesis, and low yield of n-butyllithium, and is suitable for large-scale The production and reaction conditions are mild and the process flow is short
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
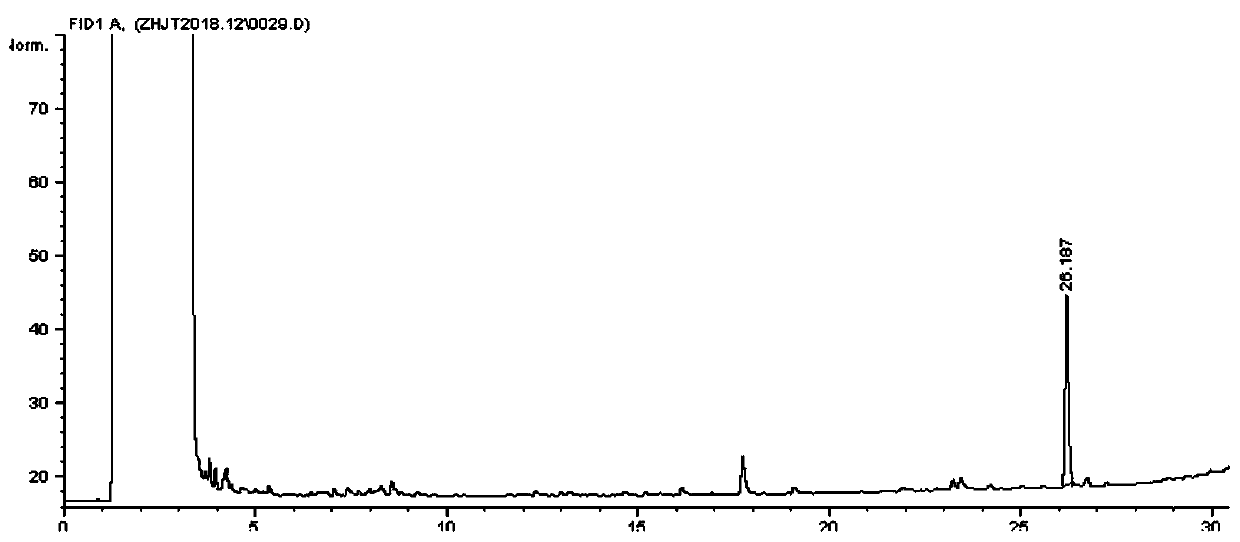
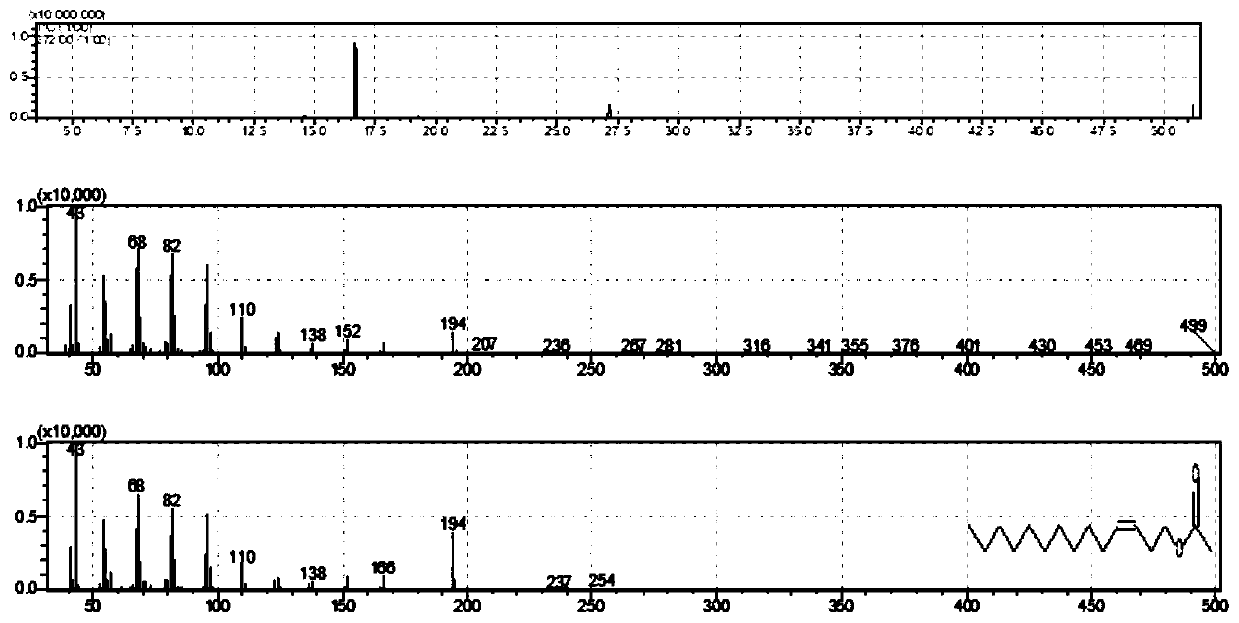
[0034] A method for synthesizing cis-3-tetradecenyl acetate and trans-3-tetradecenyl acetate as the main components of the sex attractant of the small-horned wood beetle moth, specifically comprising the following steps:
[0035] (1) Preparation of 3-tetradecynol-1
[0036] In a 500mL four-necked round-bottom flask equipped with a stirrer, reflux condenser, and constant pressure dropping funnel, put 65mL of anhydrous tetrahydrofuran and 6.2g of magnesium chips, and slowly add 35.2g of bromine under nitrogen protection and stirring. The mixture of ethane and 24mL of tetrahydrofuran was heated slightly to initiate the reaction, then cooled to 5°C, and continued to add dropwise to dissolve the magnesium chips. After the addition, it was reacted at room temperature for 2 hours to obtain a gray suspension, and then 10.22g of Add the mixture of 3-butynol-1 and 8mL tetrahydrofuran within 40 minutes, continue to react at room temperature for 3 hours, lower the temperature to 0°C, add ...
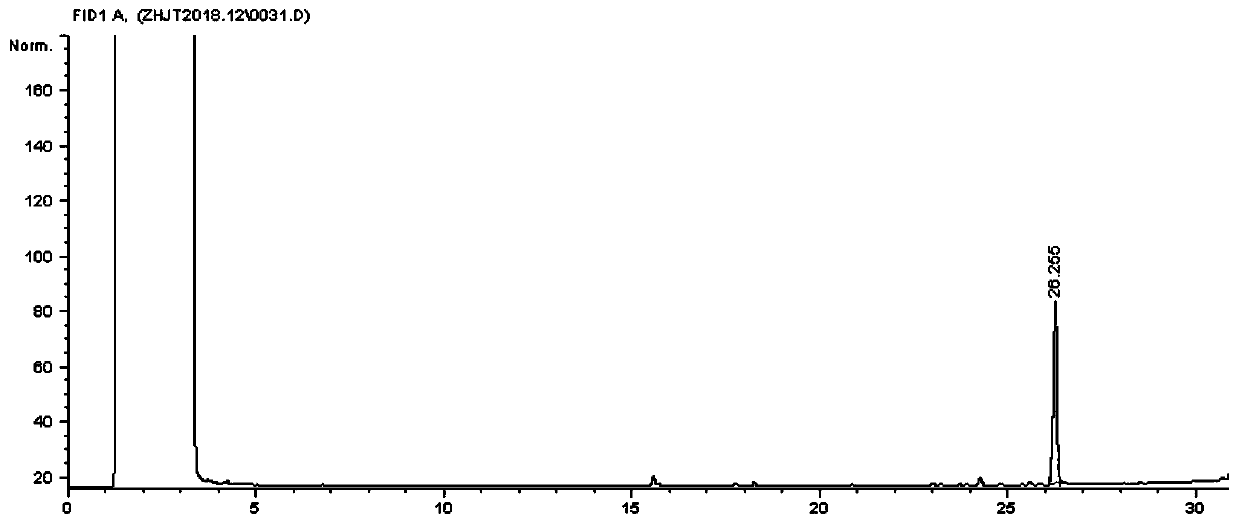
Embodiment 2
[0046] (1) Preparation of 3-tetradecynol-1
[0047] In a 1000mL four-neck round bottom flask equipped with a stirrer, reflux condenser, and constant pressure dropping funnel, put 140mL of anhydrous tetrahydrofuran and 12.5g of magnesium chips, and slowly add 70.5g of bromine under nitrogen protection and stirring. The mixed solution of ethane and 50mL tetrahydrofuran was slightly heated to initiate the reaction, then cooled to 5°C, and continued to add dropwise to dissolve the magnesium chips. After the addition, it was reacted at room temperature for 2 hours to obtain a gray suspension, and then added dropwise with 20.5g of Add the mixture of 3-butynol-1 and 15mL tetrahydrofuran within 40 minutes, continue to react at room temperature for 3 hours, cool down to 0°C, add 0.8 g of newly prepared cuprous chloride, stir for 15 minutes, the suspension turns from gray to Yellow, then dropwise add a mixture of 40.8g of 1-bromo-decane and 30mL of tetrahydrofuran, reflux at 65-70°C for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com