A kind of preparation method of iopromide
A technology of iopromide and hydroxypropylcarbamoyl, which is applied in the field of preparation of iopromide, can solve the problems of poor atom economy, increased cost of protecting groups and the like, and achieves the effects of reducing production cost and improving atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
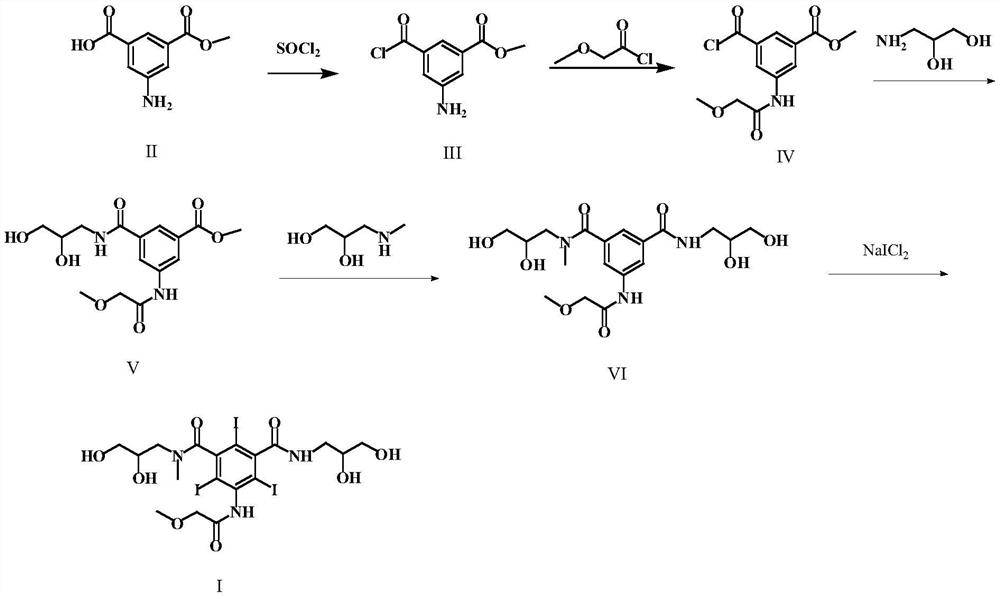
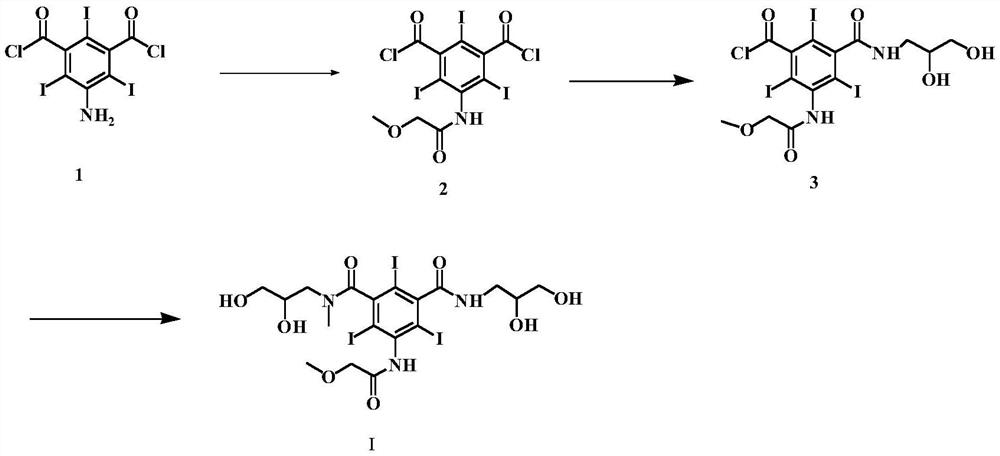
[0044] (S1) Preparation of 3-amino-5-chloroformylbenzoic acid methyl ester (formula III)
[0045] Add 10 g of 5-aminoisophthalic acid monomethyl ester (Formula II) and 20 ml of thionyl chloride into the reaction flask, and reflux for 4 hours. Distilled to dryness under reduced pressure to obtain 11.6 g of solid. The yield was 97.8%.
[0046] (S2) Preparation of 3-chloroformyl 5-[(methoxyacetyl)amino)]-benzoic acid methyl ester (formula IV)
[0047] Add 10 g of the compound of formula III, 100 ml of dioxane, and 7.1 g of methoxyacetyl chloride into the reaction flask, and stir the reaction for 6 h at room temperature. TLC detects that the reaction is complete. The solvent is evaporated under reduced pressure, and the next reaction is carried out directly.
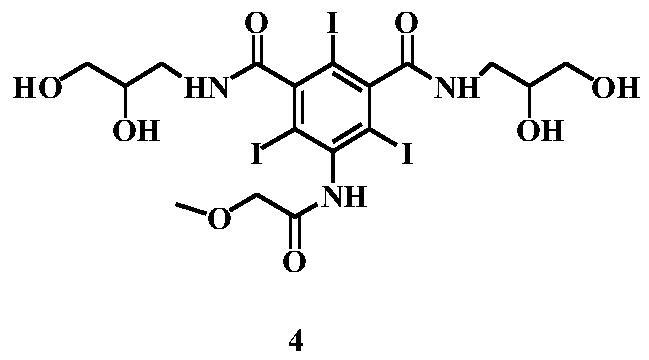
[0048] (S3) Preparation of 3-[(2,3-dihydroxypropylcarbamoyl)-5-[(methoxyacetyl)amino]-benzoic acid methyl ester (Formula V)
[0049] In the reaction flask, add the reactants of the previous step, 5.1g of 3-aminopropanedio...
Embodiment 2
[0055] (S1) Preparation of 3-amino-5-chloroformylbenzoic acid methyl ester (formula III)
[0056] Add 10 g of 5-aminoisophthalic acid monomethyl ester (formula II), 5 ml of thionyl chloride, and 50 ml of dichloromethane into the reaction flask, and reflux for 5 h. Distilled to dryness under reduced pressure to obtain 11.7 g of solid. The yield was 98.7%.
[0057] (S2) Preparation of 3-chloroformyl 5-[(methoxyacetyl)amino)]-benzoic acid methyl ester (formula IV)
[0058] Add 10 g of the compound of formula III, 30 ml of DMF, and 5.6 g of methoxyacetyl chloride into the reaction flask. Stir the reaction for 6 hours at room temperature. TLC detects that the reaction is complete. The solvent is evaporated under reduced pressure, and the next reaction is carried out directly.
[0059] (S3) Preparation of 3-[(2,3-dihydroxypropylcarbamoyl)-5-[(methoxyacetyl)amino]-benzoic acid methyl ester (Formula V)
[0060] Into the reaction bottle, add the reactants of the previous step, 6.3g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com