Synthesis method of teneligliptin-related impurity
A synthesis method and impurity technology are applied in the field of pharmaceutical synthesis to achieve the effects of easy availability of raw materials, simple operation method and reasonable process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
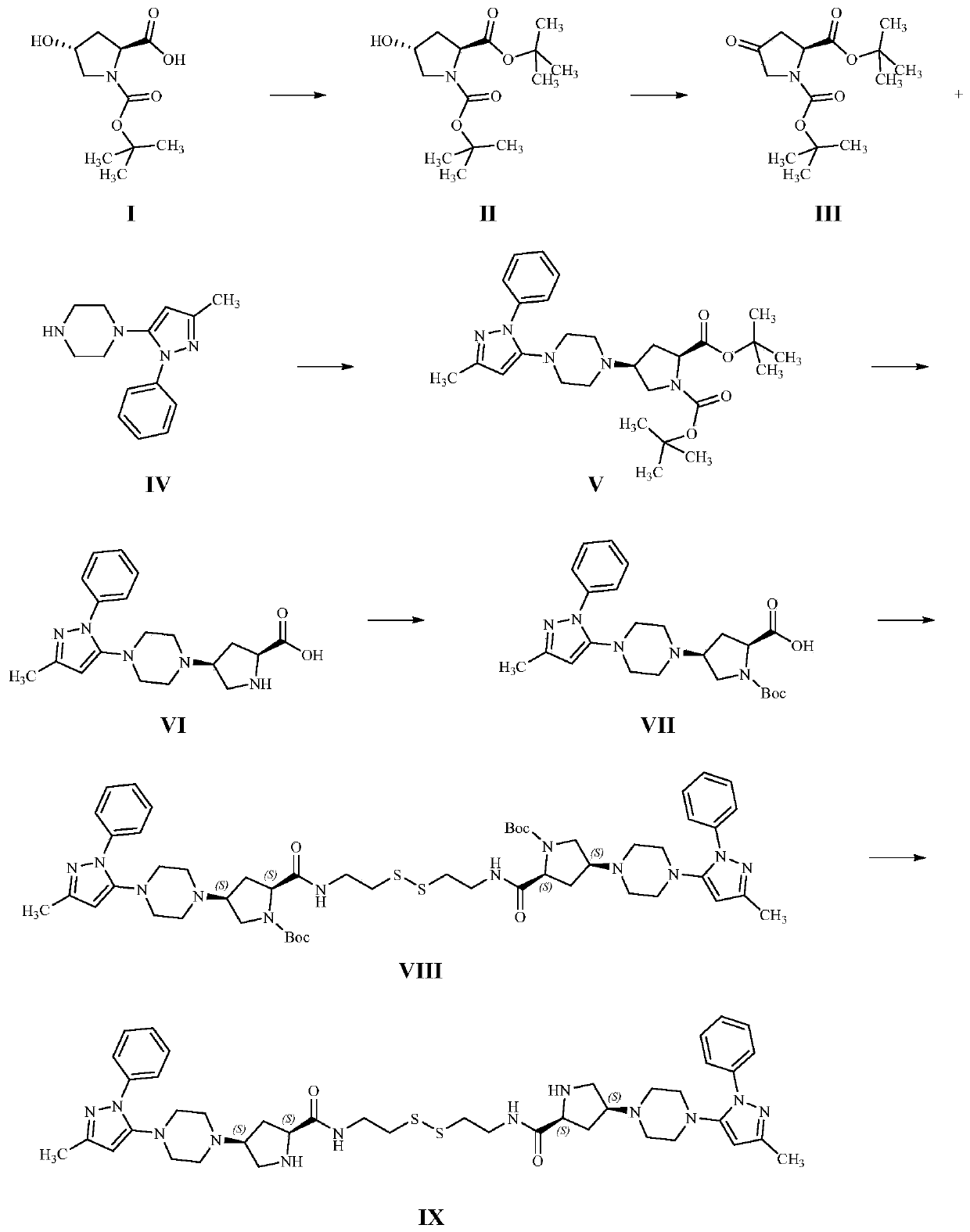
[0057] A method for synthesizing related impurities of teneligliptin, comprising the following steps:
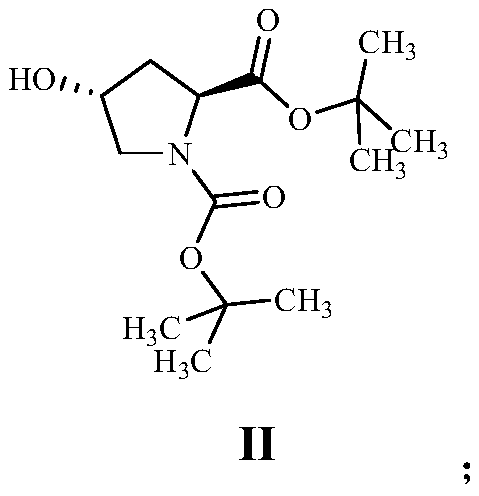
[0058] (1) Dissolve 22.50g of Boc-L-hydroxyproline in 250mL of tetrahydrofuran, and add 42.43g of O-tert-butyl-N,N'-diisopropylisourea dropwise at room temperature. After stirring for 12 hours, a white turbid liquid was obtained. The completion of the reaction was monitored by pointing a plate; the reaction liquid was filtered to remove solid insoluble matter, and the filtrate was evaporated to dryness and purified by column chromatography to obtain 21.70 g of yellow oily compound II, with a reaction yield of 82.73%.
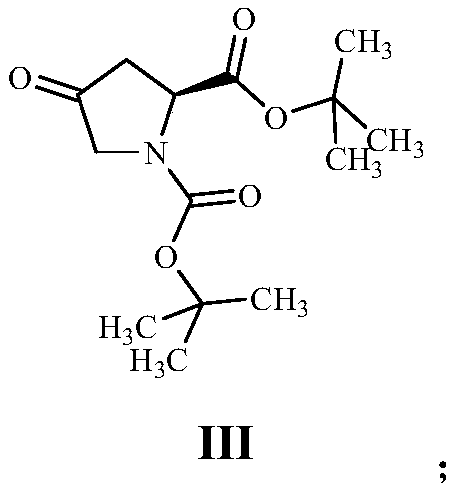
[0059] (2) Dissolve 17.36g of compound II in dichloromethane, add 9.45g of N-methyl-N-morpholine oxide and stir at room temperature for 1.5 hours, the reaction is complete to obtain a black turbid liquid, and the reaction liquid is filtered out to remove insoluble matter , the filtrate was evaporated to dryness and purified by column chromatography to obtain 1...
Embodiment 2
[0067] A method for synthesizing related impurities of teneligliptin, comprising the following steps:
[0068] (1) Dissolve 30g of Boc-L-hydroxyproline in 300mL of tetrahydrofuran, add 62.92g of O-tert-butyl-N,N'-diisopropylisourea dropwise at room temperature, and stir at 60°C After 12 hours, a white turbid solution was obtained, and the reaction was monitored by pointing a plate; the reaction solution was filtered to remove solid insoluble matter, and the filtrate was evaporated to dryness and purified by column chromatography to obtain 30.11 g of yellow oily compound II, with a reaction yield of 80.77%.
[0069] (2) Dissolve 30.11g of compound II in dichloromethane, add 5.84g of tetrapropyl ammonium perruthenate and stir at room temperature for 3.5 hours, the reaction is complete to obtain a black turbid solution, the reaction solution is suction filtered to remove insoluble matter, and the filtrate is evaporated After drying, it was purified by column chromatography to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com