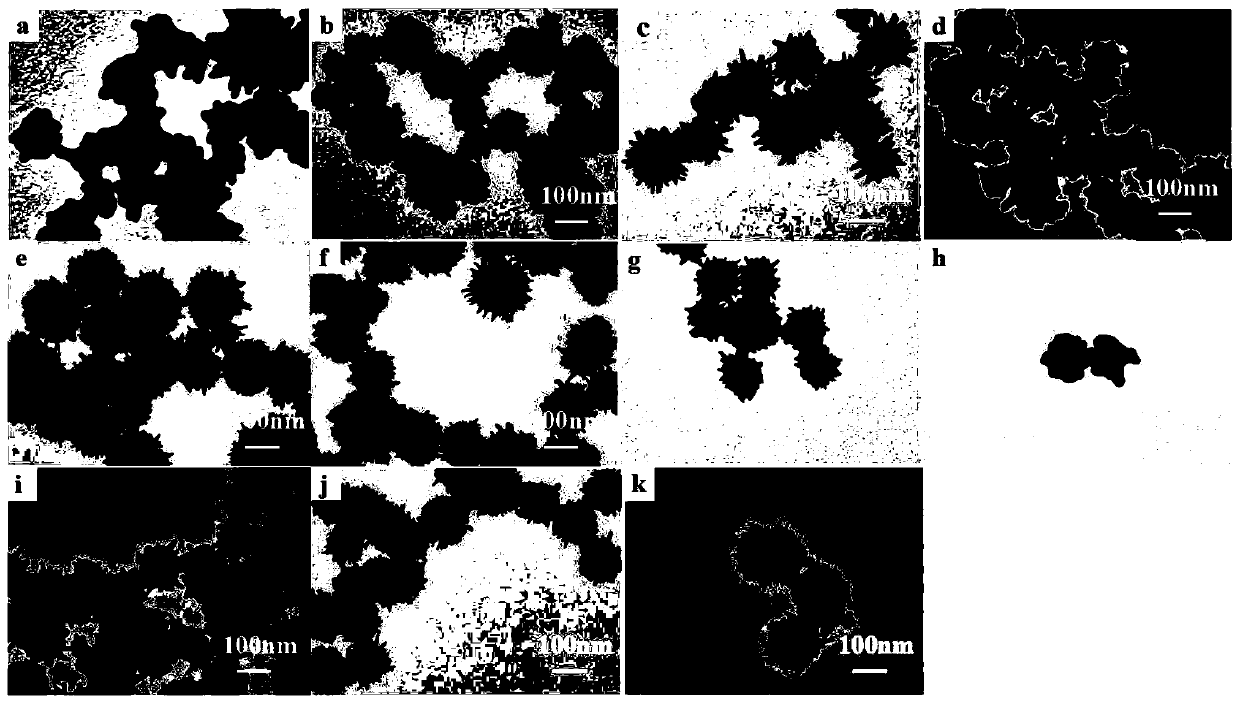
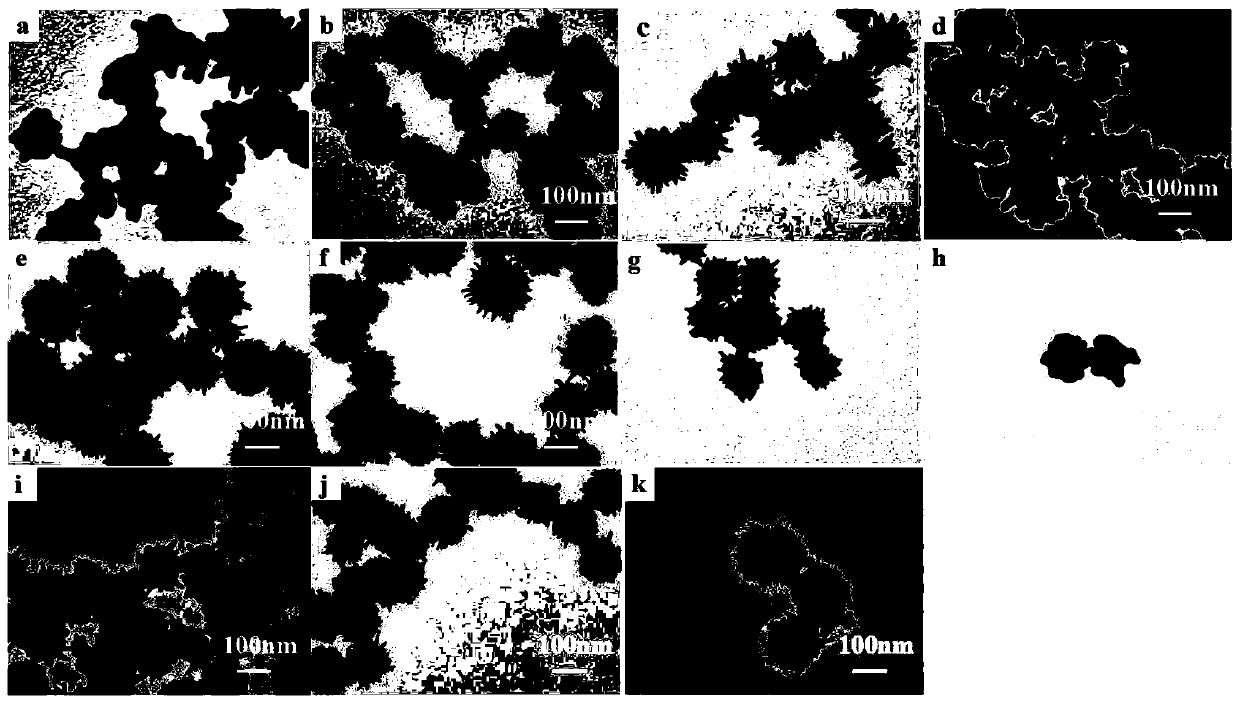
Sea-urchin-shaped gold nano particles and synthesis method thereof
A gold nanoparticle and synthesis method technology, applied in the field of sea urchin-shaped gold nanoparticle and its synthesis, to achieve the effects of good repeatability, strong shape controllability, and good application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A synthetic method of sea urchin-shaped gold nanoparticles, comprising the steps of:
[0021] (1) The required raw materials soluble silver source (silver nitrate), soluble gold source 1 (chloroauric acid), soluble gold source 2 (chloroauric acid) and weak reducing agent (ascorbic acid) were prepared into solutions using ultrapure water. Wherein, the concentration of soluble silver source (silver nitrate) solution is 0.1mol / L, the concentration of soluble gold source one (chloroauric acid) is 0.1mol / L, and the concentration of soluble gold source two (chloroauric acid) is 3mmol / L , the concentration of weak reducing agent (ascorbic acid) is 10mmol / L.
[0022] (2) Vigorously mix 2 μL soluble silver source (silver nitrate) solution and 2 μL soluble gold source-(chloroauric acid) solution in a glass bottle (the molar ratio of silver source to gold source-1 is 1:1), and quickly add 1mL of weak reducing agent (ascorbic acid) solution was vigorously stirred for 5s, then quic...
Embodiment 2
[0028] This embodiment is similar to Embodiment 1. The variables in Embodiment 1 are fixed and other variables are changed, including the following steps:
[0029] (1) The required raw materials soluble silver source (silver nitrate), soluble gold source 1 (chloroauric acid), soluble gold source 2 (chloroauric acid) and weak reducing agent (ascorbic acid) were prepared into solutions using ultrapure water. Wherein, the concentration of soluble silver source (silver nitrate) solution is 0.1mol / L, the concentration of soluble gold source one (chloroauric acid) is 0.1mol / L, and the concentration of soluble gold source two (chloroauric acid) is 3mmol / L respectively. L, the concentration of weak reducing agent (ascorbic acid) is 10mmol / L.
[0030] (2) Vigorously mix 2 μL soluble silver source (silver nitrate) solution and 8 μL soluble gold source-(chloroauric acid) solution in a glass bottle (the molar ratio of silver source to gold source-1 is 1:4), and quickly add 1mL of weak re...
Embodiment 3
[0036] This embodiment is similar to Embodiment 1 and 2, fixing the variables in Embodiment 1 and 2, changing other variables, including the following steps:
[0037] (1) The required raw materials soluble silver source (silver nitrate), soluble gold source 1 (chloroauric acid), soluble gold source 2 (chloroauric acid) and weak reducing agent (ascorbic acid) were prepared into solutions using ultrapure water. Wherein, the concentration of soluble silver source (silver nitrate) solution is 0.1mol / L, the concentration of soluble gold source one (chloroauric acid) is 0.1mol / L, and the concentration of soluble gold source two (chloroauric acid) is 0mmol / L respectively L, 1mmol / L, 2mmol / L, 3mmol / L, 4mmol / L, the concentration of the weak reducing agent (ascorbic acid) is 10mmol / L.
[0038] (2) Vigorously mix 2 μL soluble silver source (silver nitrate) solution and 2 μL soluble gold source-(chloroauric acid) solution in a glass bottle (the molar ratio of silver source to gold source-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com