Preparation method of iodide ionic ligand PbS nanocrystal, iodide ionic ligand PbS nanocrystal ink and solar battery
A technology of solar cells and nanocrystals, which is applied in semiconductor/solid-state device manufacturing, circuits, photovoltaic power generation, etc., can solve problems hindering the industrialization of nanocrystal devices, and achieve the effect of reducing preparation costs, simple and fast methods, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of iodide ligand PbS nanocrystals:
[0029] 1) Preparation of precursor solution: Add 3.568 g (8 mmol) of lead iodide, 912 mg (4 mmol) of N,N-diphenylthiourea and 9 mL of N,N-dimethylformamide into a 50 mL single port In the flask, stir at room temperature until the precursor is completely dissolved to obtain a precursor solution for use; the molar ratio of lead iodide to N,N-diphenylthiourea is 2:1;
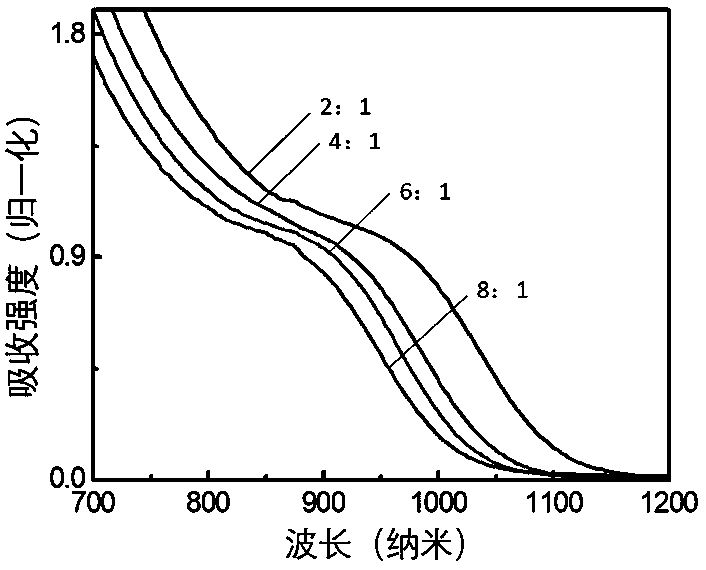
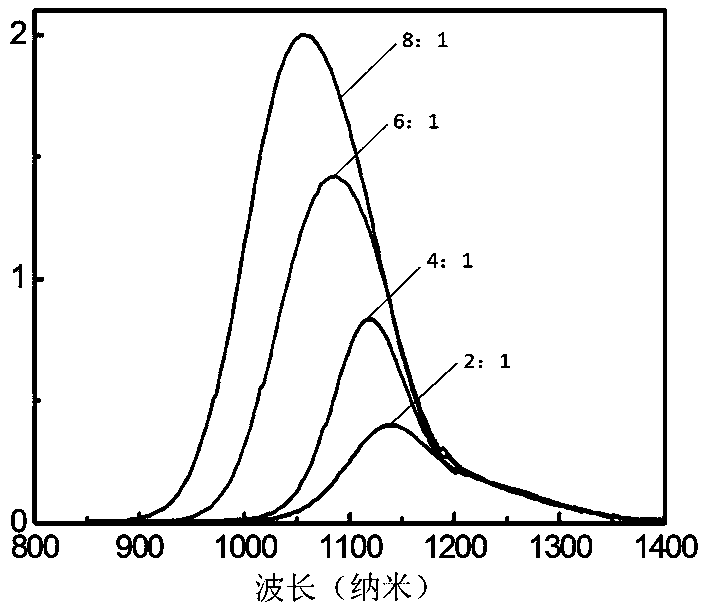
[0030] 2) Inject 1mL of butylamine into the one-necked flask, and continue stirring for 10 minutes. Transfer the reaction solution to a centrifuge tube, add toluene until the reaction solution becomes turbid, centrifuge at a speed of 8000 rpm, and discard the supernatant after centrifugation for 5 minutes; drain the product under vacuum to obtain the iodide ion ligand PbS nano Crystals, stored in the glove box. Its UV-Vis absorption spectrum is as figure 1 As shown, the photoluminescence spectrum as figure 2 shown.
Embodiment 2
[0032] Preparation of iodide ligand PbS nanocrystals:
[0033] 1) Preparation of precursor solution: Add 3.568 g (8 mmol) of lead iodide, 456 mg (2 mmol) of N,N-diphenylthiourea and 9 mL of N,N-dimethylformamide into a 50 mL single port In the flask, stir at room temperature until the precursor is completely dissolved to obtain a precursor solution for use; the molar ratio of lead iodide to N,N-diphenylthiourea is 4:1;
[0034] 2) Inject 1mL of butylamine into the one-necked flask, and continue stirring for 10 minutes. Transfer the reaction solution to a centrifuge tube, add toluene until the reaction solution becomes turbid, centrifuge at a speed of 8000 rpm, and discard the supernatant after centrifugation for 5 minutes; drain the product under vacuum to obtain the iodide ion ligand PbS nano Crystals, stored in the glove box. Its UV-Vis absorption spectrum is as figure 1 As shown, the photoluminescence spectrum as figure 2 shown.
Embodiment 3
[0036] Preparation of iodide ligand PbS nanocrystals:
[0037] 1) Preparation of precursor solution: Add 3.568 g (8 mmol) of lead iodide, 304 mg (1.33 mmol) of N,N-diphenylthiourea and 9 mL of N,N-dimethylformamide into a 50 mL single port In the flask, stir at room temperature until the precursor is completely dissolved to obtain a precursor solution for use; wherein the molar ratio of lead iodide to N,N-diphenylthiourea is 6:1;
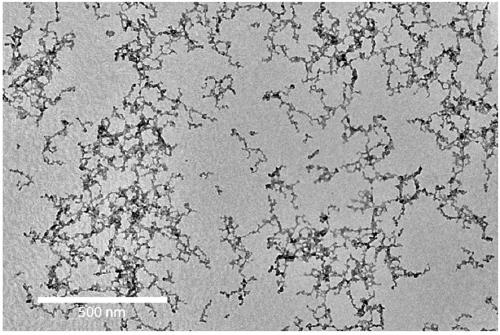
[0038] 2) Inject 1mL of butylamine into the one-necked flask, and continue stirring for 10 minutes. Transfer the reaction solution to a centrifuge tube, add toluene until the reaction solution becomes turbid, centrifuge at a speed of 8000 rpm, and discard the supernatant after centrifugation for 5 minutes; the product is dried under vacuum to obtain the iodide ion ligand PbS nano Crystals, stored in the glove box. Its UV-Vis absorption spectrum is as figure 1 As shown, the photoluminescence spectrum as figure 2 As shown, the transmission electr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com