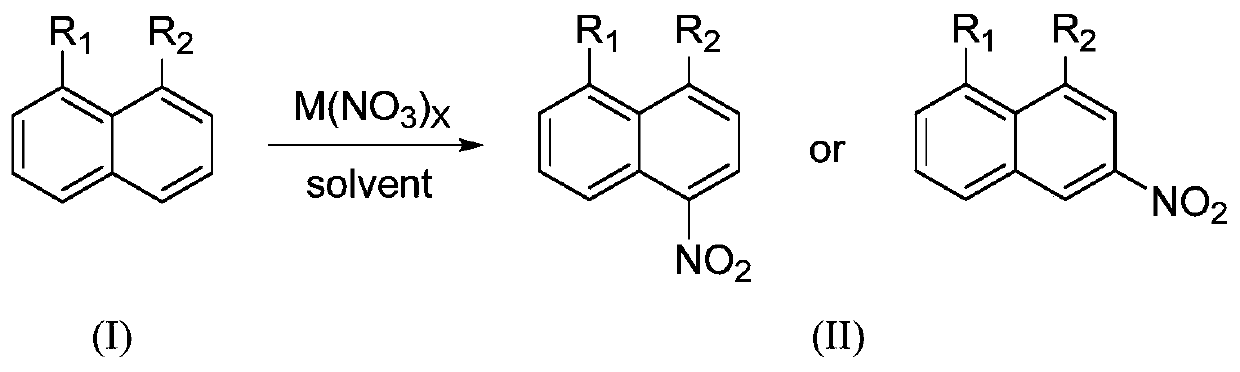
Preparation method of 1,8-disubstituted naphthalene series polycyclic aromatic hydrocarbon mononitration derivative
A fused-ring aromatic hydrocarbon and disubstituted technology, which is applied in the field of preparation of 1,8-disubstituted naphthalene-based condensed-ring aromatic hydrocarbon mononitration derivatives, can solve problems such as poor selectivity, poor substrate universality, and many wastes, and achieve low cost Low, high product yield and high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
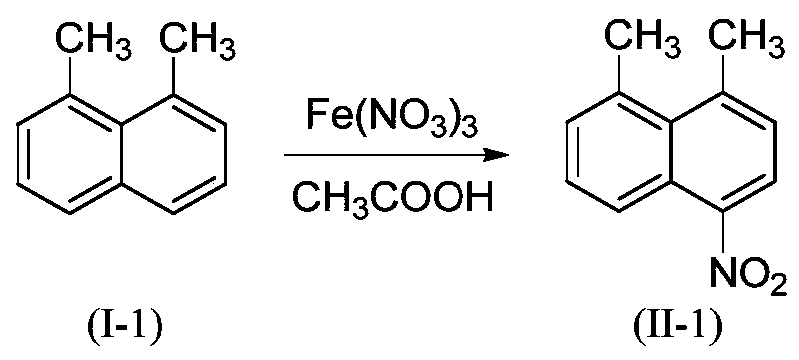
Embodiment 1
[0026]
[0027] 3.12g (20mmol, M=156.23) 1,8-dimethylnaphthalene (I-1), 9.29g (23 mmol, M=404.00) Fe(NO 3 ) 3 9H 2 O was added to 26mL CH 3 In COOH, the temperature was controlled at 40°C for 5 hours, and the end point of the reaction was monitored by TLC until the disappearance of the raw material point. Cool to room temperature, filter with suction, and use a small amount of H 2 O and anhydrous C 2 h 5 The filter cake was washed with OH and vacuum-dried to obtain the light yellow product 4-nitro-1,8-dimethylnaphthalene (II-1) with a yield of 92%. HPLC purity 98.6%. HRMS(ES+)C 12 h 12 NO 2 ([M+H]) + The theoretical value is 202.0868, and the measured value is 202.0866.
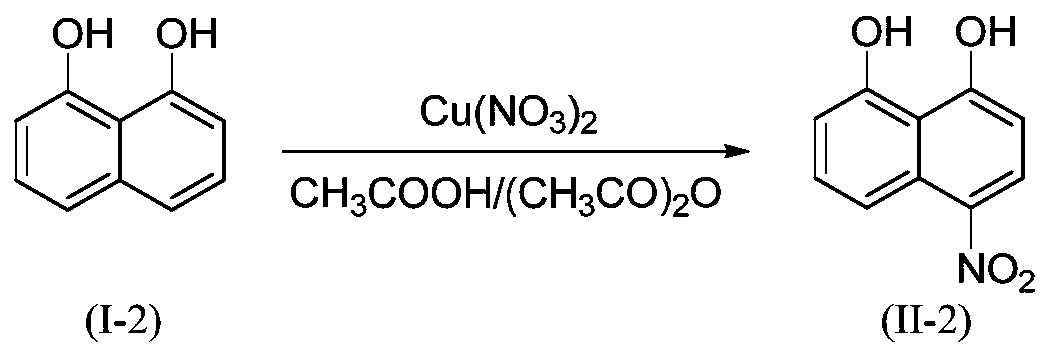
Embodiment 2
[0029]
[0030] 3.20g (20mmol, M=160.17) 1,8-dihydroxynaphthalene (I-2), 5.35g (23 mmol, M=232.59) Cu(NO 3 ) 2 2.5H 2 O was added to 13mL CH 3 COOH+13 mL(CH 3 CO) 2 In O, the temperature was controlled at 25°C for 5 h. Other conditions and preparation steps were the same as in Example 1 to obtain the orange-yellow product 4-nitro-1,8-naphthalenediol (II-2) with a yield of 91%. HPLC purity 99.2%. HRMS(ES+)C 10 h 8 NO 4 ([M+H]) + The theoretical value is 206.0453, and the measured value is 206.0453.
Embodiment 3
[0032]
[0033] 3.16g (20mmol, M=158.20) 1,8-diaminonaphthalene (I-3), 9.29g (23 mmol, M=404.00) Fe(NO 3 ) 3 9H 2 O was added to 13mL CH 3 COOH+13mL (CH 3 CO) 2 In O, the temperature was controlled at 25°C for 5 h. Other conditions and preparation steps were the same as in Example 1 to obtain the orange-red product 4-nitro-1,8-naphthalenediamine (II-3) with a yield of 94%. HPLC purity 99.0%. HRMS(ES+)C 10 h 10 N 3 o 2([M+H]) + The theoretical value is 204.0773, and the measured value is 204.0774.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com