Synthesis method of pyroxasulfone
A synthetic method, the technology of pyrazazone sulfone, which is applied in the field of synthesis of pyrazone sulfone, can solve the problems of many wastes in the preparation process, low yield and purity, high cost, etc., and achieve shortening of reaction time, product yield and The effect of high purity and easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
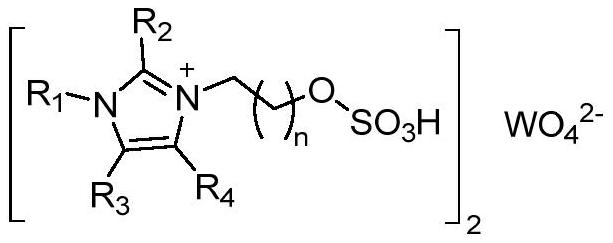
[0053] The preparation of embodiment 1 ionic liquid 4a
[0054]
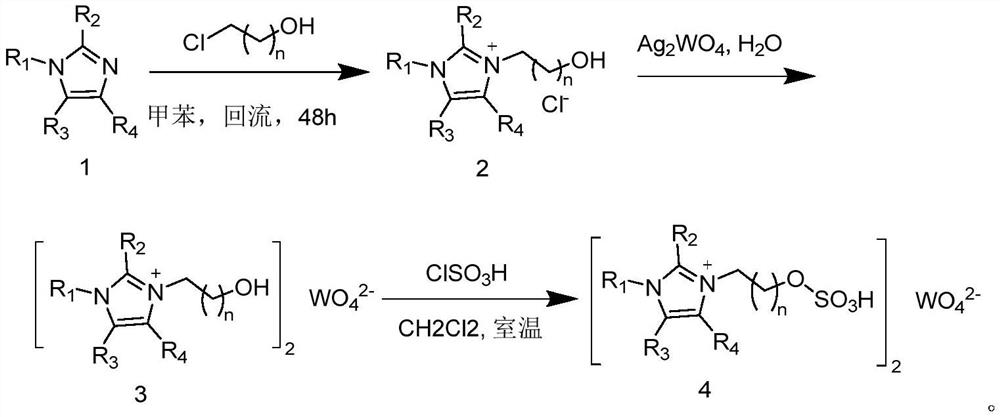
[0055] 1) Add (8.2 g, 0.10 mol) 1-methylimidazole (1a) to a solution of 2-chloroethanol (7.4 ml, about 0.11 mol) in anhydrous toluene (50 mL), and stir at reflux temperature for 48 hours , the solvent was poured out and washed with ether, and the ionic liquid 2a was obtained after vacuum drying;
[0056] 2) Add (0.026mol) Ag to (8.1g, 0.05mol) aqueous solution of ionic liquid 2a at room temperature 2 WO 4 , after stirring for 1 h, the reaction mixture was filtered, the filtrate was concentrated, and vacuum-dried to obtain ionic liquid 3a in the form of a colorless liquid;
[0057] 3) Add 30 mL of dichloromethane to (2.5 g, 5 mmol) ionic liquid 3a, stir evenly, at room temperature, add (1.22 g, 10.5 mmol) chlorosulfonic acid dropwise to the above mixture within 10 minutes, and stir the reaction mixture After 30-60 minutes, the dichloromethane was removed and the mixture was washed with anhydrous dichloromet...
Embodiment 2-5
[0058] Example 2-5 Preparation of ionic liquids 4b-4e
[0059] With reference to the method of Example 1, ionic liquids 4b-4e were prepared respectively:
[0060]
Embodiment 6
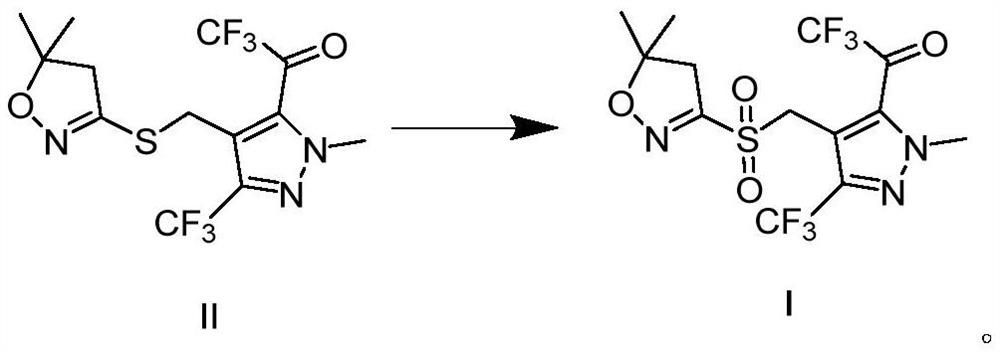
[0061] The preparation of embodiment 6 sulfofenapyr
[0062]
[0063] In a 300ml three-necked flask, 80mL of methanol was added, and then 38.9g (0.1mol) of compound II was added. After stirring and dissolving, (1.32g, 2mmol) acidic ionic liquid 4a was added, and then (0.5mol) 30% hydrogen peroxide was added dropwise, and the reaction was stirred at room temperature. 3h. After the reaction finishes, dripping concentration is 10% sodium thiosulfate aqueous solution in the system to quench excessive hydrogen peroxide, filter, dry, obtain 40.2g white solid fenfenpyr I, yield 95.5%, use liquid chromatography ( LC) detected that the content of sulfofenapyr was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com