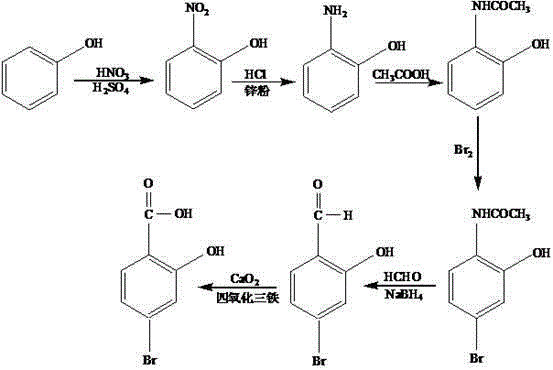
Synthesis method of 2-hydroxyl-4-bromobenzoic acid
A technology of bromobenzoic acid and a synthetic method is applied in the synthesis field of 2-hydroxy-4-bromobenzoic acid, can solve problems such as high cost, low production capacity, environmental pollution, etc., and achieves simple operation, improved product yield and purity , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] First get 60g of phenol and put it into a 500mL three-neck flask equipped with a stirrer, a thermometer and a dropping funnel, add 50mL of nitric acid with a mass fraction of 69%, control the rotating speed at 500r / min, and stir at a temperature of 10°C. During the stirring process, slowly add 30mL of sulfuric acid with a mass fraction of 75% dropwise, and control the dropwise addition within 15min. After the dropwise addition is completed, raise the temperature to 20°C and set the pressure to 1MPa, and react for 40min to obtain 2-nitro Phenol; then the 2-nitrophenol obtained above is placed in a reaction kettle, 15 mL of hydrogen chloride with a mass fraction of 30% is added thereto, after stirring evenly, after adding 0.5 g of zinc powder, at a temperature of 60 ° C, a pressure Under the condition of 0.8MPa, hydrogen gas was introduced, and the reactor was sealed, and the reaction was continued for 1h to obtain 2-aminophenol; then 10mL of CH with a mass fraction of 37%...
example 2
[0022] First get 100ml of phenol and put it into a 500mL three-necked flask equipped with a stirrer, a thermometer and a dropping funnel, add 55mL of nitric acid with a mass fraction of 69%, control the rotating speed at 550r / min, and stir at a temperature of 13°C. During the stirring process, slowly add 35 mL of sulfuric acid with a mass fraction of 75% dropwise, and control the dropwise addition within 18 minutes. After the dropwise addition is completed, raise the temperature to 23°C and set the pressure at 1.1MPa. React for 43 minutes to obtain 2-nitrate Then put the 2-nitrophenol obtained above in the reaction kettle, add 16mL of hydrogen chloride with a mass fraction of 30% to it, stir evenly, and then add 0.7g of zinc powder, at a temperature of 65°C, Under the condition of a pressure of 1.2MPa, hydrogen gas was introduced, and the reactor was sealed, and the reaction was continued for 1.3h to obtain 2-aminophenol; then 15mL of CH with a mass fraction of 37% was taken 3...
example 3
[0025] First get 120g of phenol and put it into a 500mL three-necked flask equipped with a stirrer, a thermometer and a dropping funnel, add 60mL of nitric acid with a mass fraction of 69% therein, control the rotating speed at 600r / min, and stir at a temperature of 15°C. During the stirring process, slowly add 40mL of sulfuric acid with a mass fraction of 75%, and control the dropwise addition within 20min. After the dropwise addition is completed, raise the temperature to 25°C, set the pressure at 1.2MPa, and react for 45min to obtain 2-nitrate base phenol; then the 2-nitrophenol obtained above is placed in a reaction kettle, 18 mL of hydrogen chloride with a mass fraction of 30% is added thereto, after stirring evenly, after adding 0.8 g of zinc powder, at a temperature of 70 ° C, Under the condition of a pressure of 1.3MPa, hydrogen gas was introduced, and the reactor was sealed, and the reaction was continued for 1.5h to obtain 2-aminophenol; then 18mL of CH with a mass fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com