Magnesium picolinate compositions and methods of use
A technology of magnesium picolinate and picolinic acid, which can be used in drug combination, drug delivery, pharmaceutical formulation, etc., and can solve problems such as shortage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Attempt to prepare magnesium picolinate according to Swiderski
[0101] According to Swiderski, MgPic can be prepared by the exchange reaction of magnesium sulfate and barium picolinate. Therefore, a barium picolinate solution was prepared by dissolving 2 molar equivalents of picolinic acid and 1 molar equivalent of barium hydroxide in 100 mL of water. When a clear solution of barium picolinate was obtained, 1 molar equivalent of magnesium sulfate powder was added. After stirring for 12 h, the slurry was filtered to remove solid barium sulfate, the filtrate was concentrated to dryness, and the resulting solid was dried at 120 °C for 24 h to obtain anhydrous MgPic. The barium level in the anhydrous MgPic was found to be greater than 200 ppm. This far exceeds the drug tolerance of about 200 ppm barium contamination. Repeated dissolution and crystallization attempts to reduce barium contamination levels resulted in a loss of almost 50% of the MgPic and failed...
Embodiment 2
[0102] Example 2: Preparation A of crystalline and amorphous magnesium picolinate
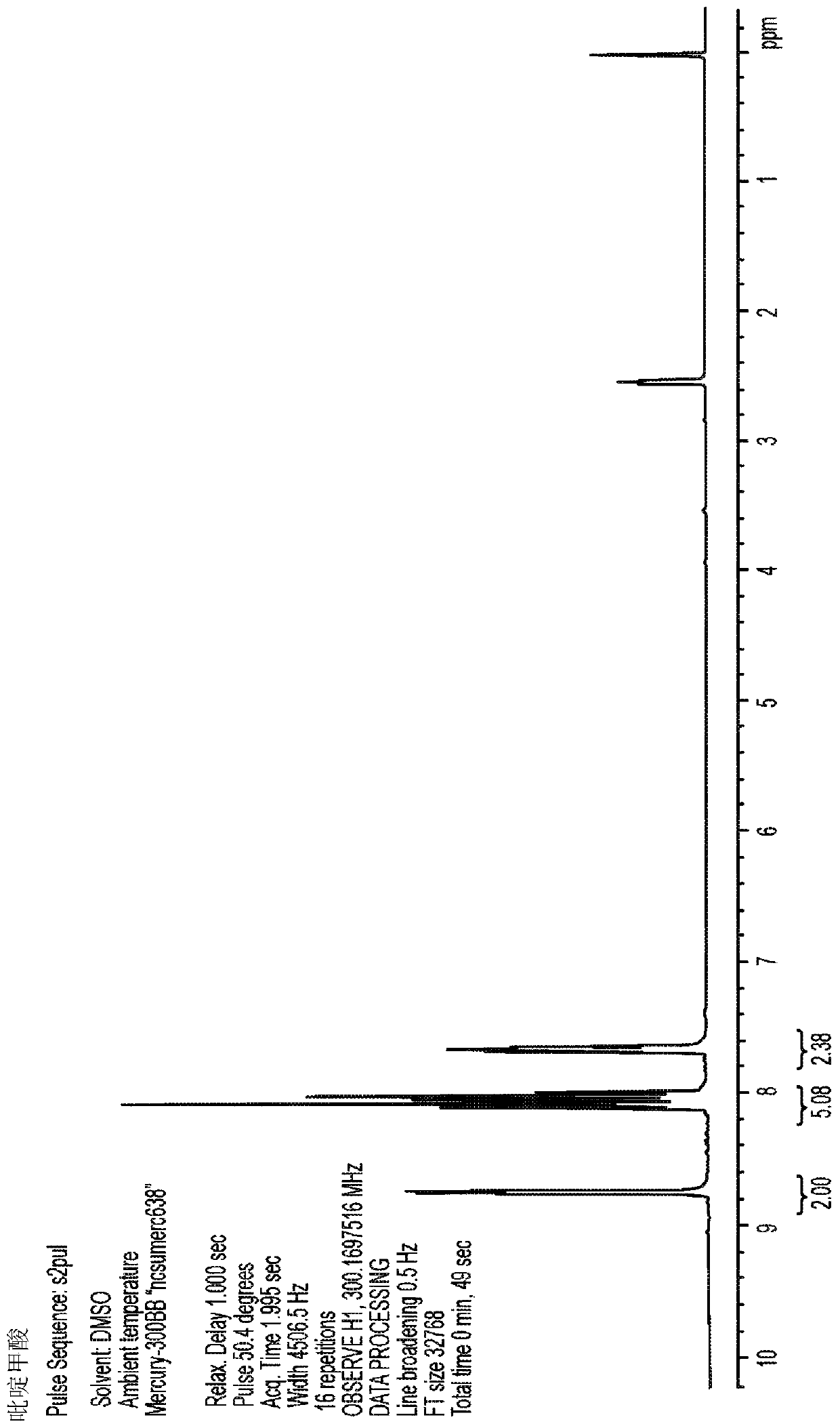
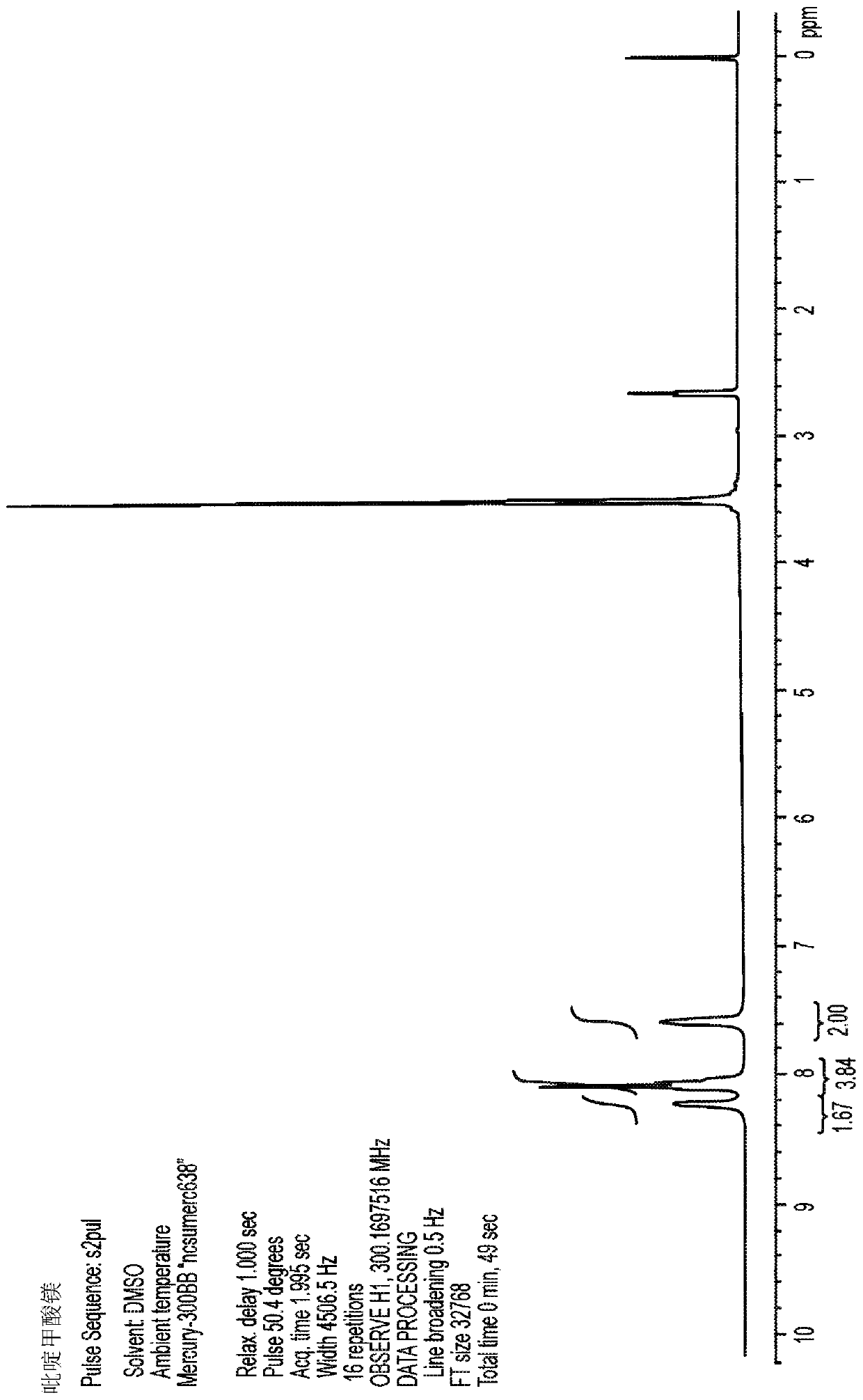
[0103] Picolinic acid (3.7 g, 30 mmol) and magnesium acetate tetrahydrate (3.2 g, 15 mmol) were suspended in 25 mL of water. The slurry was stirred and heated at reflux overnight, then filtered to isolate 1.7 g of crystalline MgPic. The remaining filtrate was cooled and evaporated to dryness, affording an additional 2.2 g of amorphous MgPic with traces of residual acetate. Acetate was removed by washing the solid with ethanol to provide MgPic (crystalline and amorphous) in 96% overall yield. product of 1 H-NMR spectrum ( figure 2 ) and the spectrum of picolinic acid metal salt Figure 1 Sincerely. The product is readily soluble in water and provides a clear and colorless MgPic solution. This product contains acceptable traces of barium and other heavy metals at levels of less than 200ppm for each element tested. Accordingly, embodiments of the MgPic composition may contain less than abo...
Embodiment 3
[0104] Embodiment 3: Preparation B of crystalline magnesium picolinate
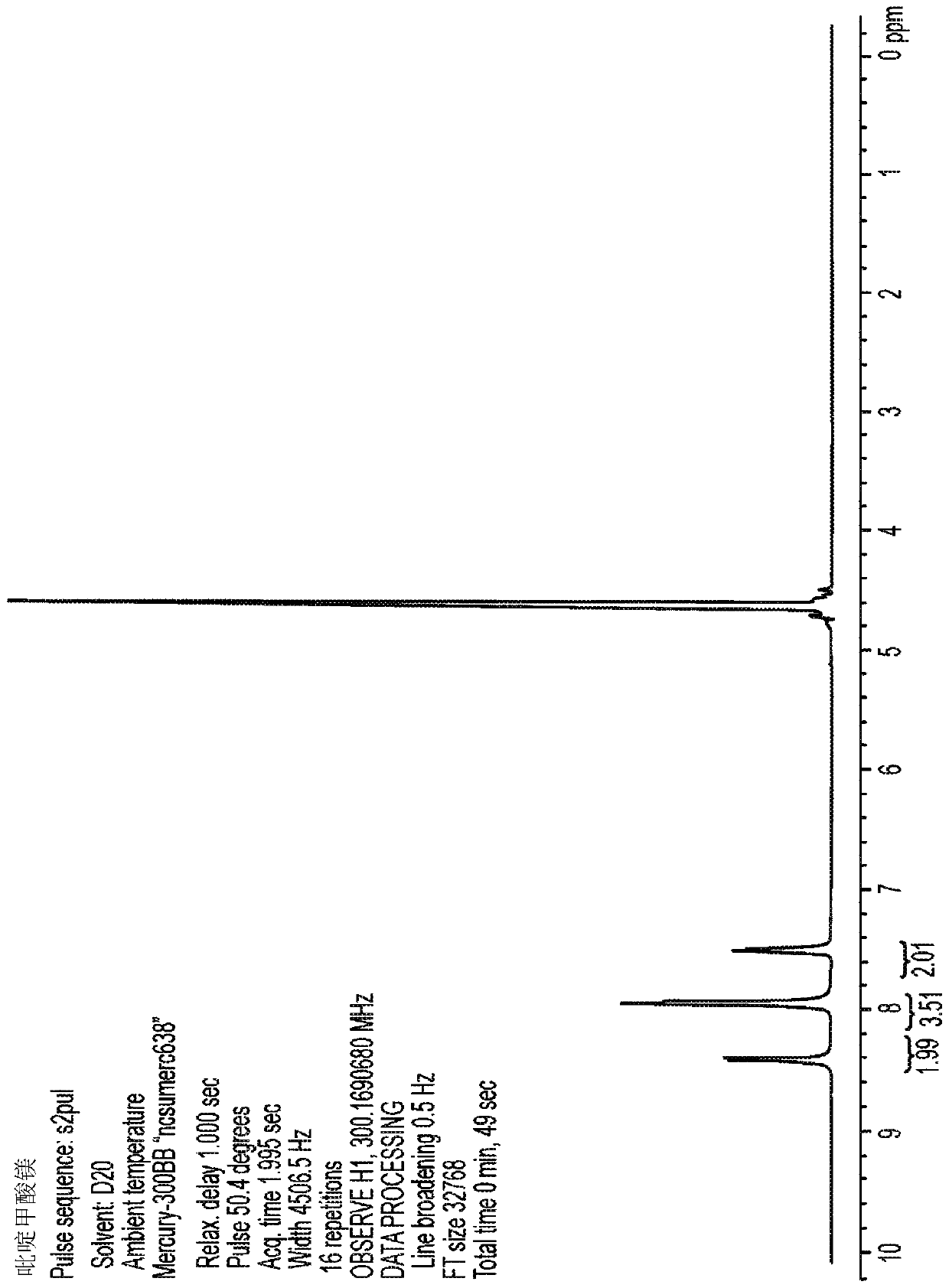
[0105] Picolinic acid (1.23 g, 10 mmol) was added to 25 mL of ethanol and stirred at room temperature until complete dissolution was observed. Magnesium ethoxide (560 mg, 4.9 mmol) was added and the slurry was heated to 80°C. Water was added dropwise until a clear solution was obtained. The solution was filtered while hot, allowed to cool to room temperature, and then kept at 4°C overnight. The resulting white crystals were isolated by filtration, washed with ethanol, and air-dried to afford 1.1 g of crystalline MgPic in 85% yield. product of 1 H-NMR spectrum ( image 3 ) and the spectrum of picolinic acid metal salt Figure 1 Sincerely. This product contains acceptable traces of barium and other heavy metals at levels of less than 200ppm for each element tested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com