Novel IDO inhibitor, preparation method, pharmaceutical composition and use thereof
A compound and pharmaceutical technology applied in the field of IDO inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
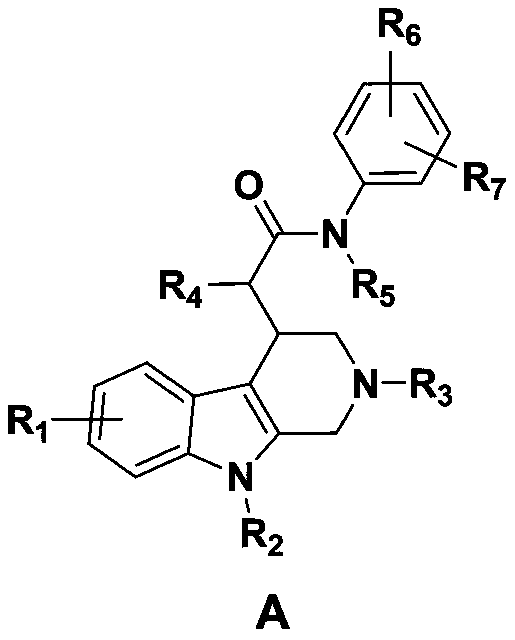
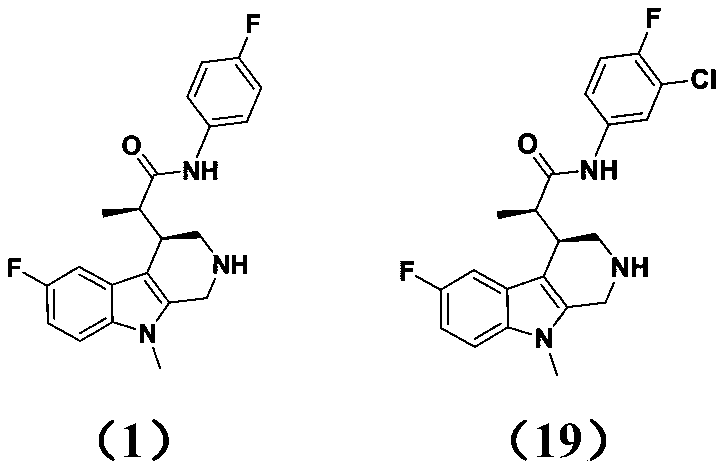
[0091] (1) The substance represented by the structure of formula (1) in the present invention: 2-(6-fluoro-9-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole The preparation method of -4-yl)-N-(4-fluorophenyl) propanamide
[0092]
[0093] step 1:
[0094] Put p-fluorophenylhydrazine 1a (75.68g, 600mmol), 1-benzylpiperidine-3,5-dione 1b (121.94g, 600mmol), acetic acid (600ml), trifluoroacetic acid (300ml) in a 2L reaction flask In the process, heat up to system reflux, stir and react for 8 hours, TLC monitors the reaction, after the reaction is completed, cool to room temperature, add 500ml of ethyl acetate and 200ml of water, stir and stand for layering, and extract the water layer twice with 100ml of ethyl acetate, The organic layers were combined, heated and concentrated, and separated by column chromatography to obtain 148.00 g of an off-white solid (Intermediate 1c), with a yield of 83.8%.
[0095] Step 2:
[0096] The compound 1c (117.73g, 400mmol) and potassium ca...
Embodiment 2
[0110] Preparation of compound 2: 3N-(4-chlorophenyl)-2-(6-fluoro-9-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]ind Indol-4-yl)propionamide
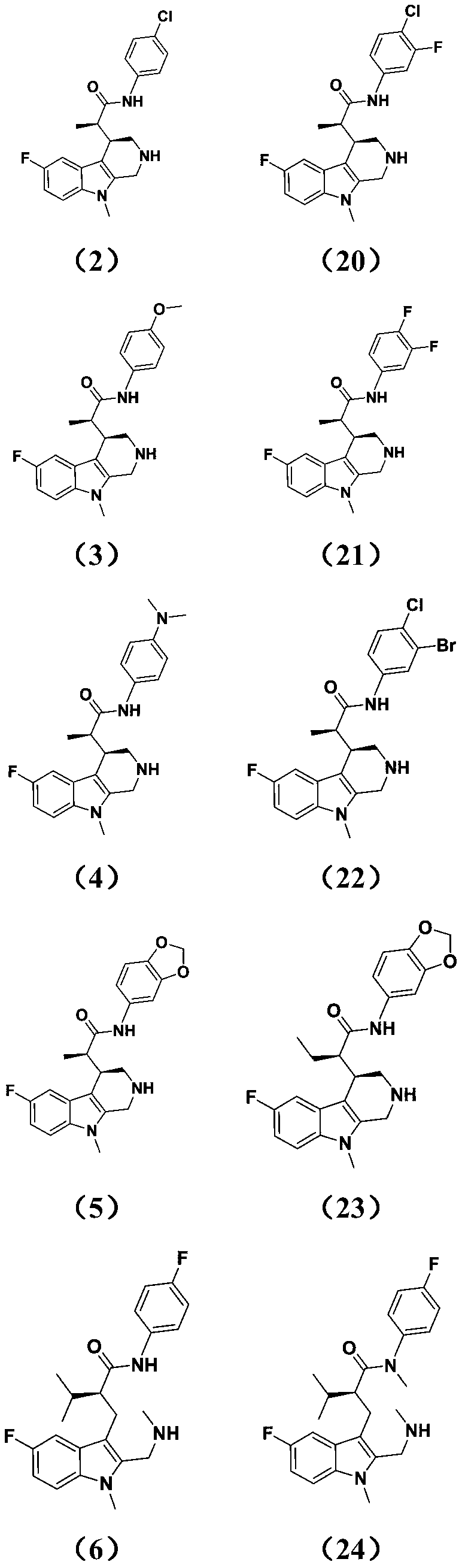
[0111] The preparation method of intermediate 1i is the same as that of Example 1, except that compound 1i (3.76g, 10mmol), p-chloroaniline (1.56g, 11mmol) and diisopropylethylamine (1.94 g, 15mmol) was dissolved in DMF (50ml), HATU (5.70g, 12mmol) was added at 25°C, the reaction was incubated, and the reaction was monitored by TLC. After the reaction was completed, water was added to quench the reaction, extracted with ethyl acetate (100ml), and the organic layer was dried , concentrated, and separated by column chromatography to obtain 3.18 g of off-white solid with a yield of 65.4%.
[0112] The intermediate obtained above (3.00g, 6.2mmol) was dissolved in dichloromethane (20ml), and trifluoroacetic acid (10ml) was added at 25°C, stirred at room temperature for 4 hours, the reaction was stopped, and the solvent and trifluoroacetic acid...
Embodiment 3
[0114] Preparation of compound 3: 2-(6-fluoro-9-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-4-yl)-N-( 4-methoxyphenyl)propionamide
[0115] The preparation method of intermediate 1i is the same as in Example 1, except that compound 1i (3.76g, 10mmol), p-methoxyaniline (1.35g, 11mmol) and diisopropylethylamine obtained in Example 1 were (1.94g, 15mmol) was dissolved in DMF (50ml), and HATU (5.70g, 12mmol) was added at 25°C, the reaction was incubated, and the reaction was monitored by TLC. After the reaction was completed, water was added to quench the reaction, extracted with ethyl acetate (100ml), organic The layer was dried, concentrated, and separated by column chromatography to obtain 2.95 g of off-white solid with a yield of 61.3%.
[0116] The intermediate obtained above (2.50g, 5.19mmol) was dissolved in dichloromethane (20ml), and trifluoroacetic acid (10ml) was added at 25°C, stirred at room temperature for 4 hours, the reaction was stopped, and the solvent and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com