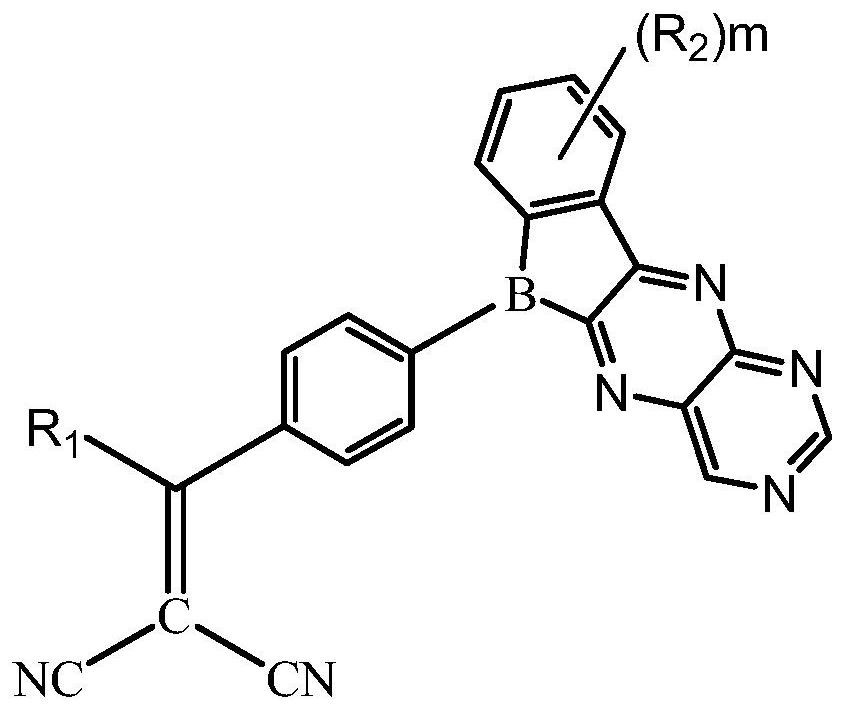
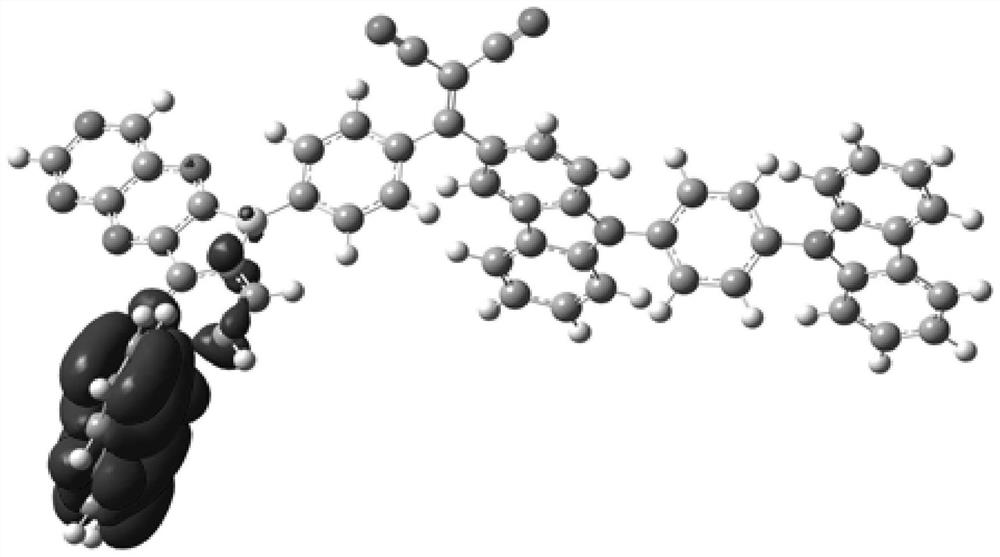
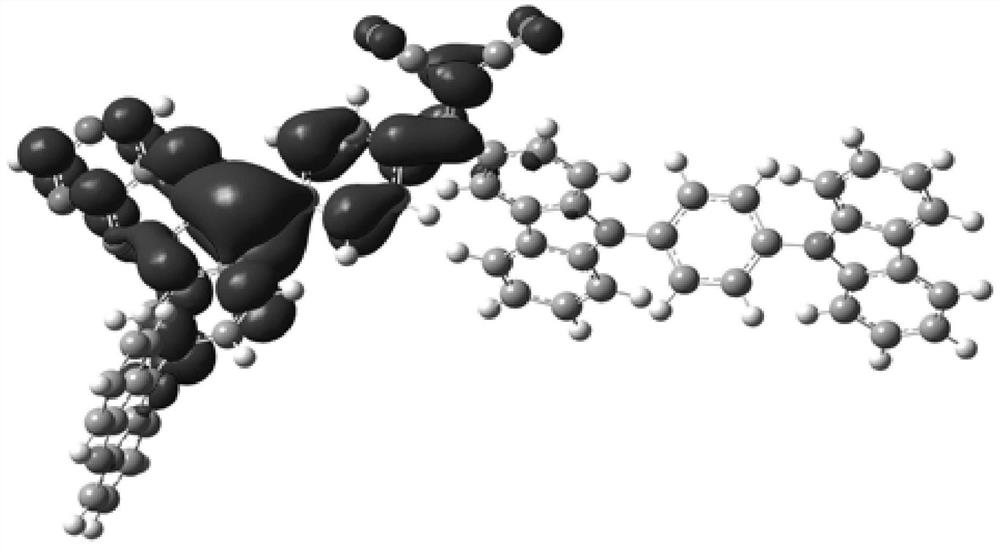
Compound, display panel and display device
A display panel and compound technology, which is applied in the field of display panels, display devices, and compounds, can solve the problems of unsatisfactory luminescence wavelength, weakened planarity, and poor overlap of luminescence spectrum, so as to improve the utilization rate of excitons, reduce device voltage, Effect of Improving Fluorescence Quantum Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Synthesis of Compound M1
[0077] The synthetic route of compound M1 is as follows.
[0078]
[0079] The specific synthesis steps of compound M1 are as follows.
[0080]
[0081] In a 200ml three-necked flask, 8.84g (20mmol) of substrate A (20mmol) and THF (80mL) were added to dissolve, and nitrogen replacement was performed three times. Cool down to -78°C, and when the temperature reaches, control the temperature below -65°C and slowly add n-BuLi 20mL (50mmol) dropwise, and stir for 30min after the dropwise addition is complete. Then 4.35 g (40 mmol) of TMS-Cl was slowly added dropwise, and the temperature was raised to 0° C. for 4 h. After completion, add ice water to quench. DCM (80mL*2) was added for extraction. The collected organic phase was rotary evaporated to obtain a pale yellow oil. Crystallization using Tol / EtOH afforded a pale yellow solid. 6.45 g (15 mmol) of light yellow solid, anhydrous toluene solution (70 mL) and 0.76 mL (8 mmol) of boron ...
Embodiment 2
[0097] Synthesis of compound M2
[0098]
[0099] The specific synthesis steps of compound M2 are as follows.
[0100]
[0101] In a 200ml three-necked flask, 8.84g (20mmol) of substrate A (20mmol) and THF (80mL) were added to dissolve, and nitrogen replacement was performed three times. Cool down to -78°C, and when the temperature reaches, control the temperature below -65°C and slowly add n-BuLi 20mL (50mmol) dropwise, and stir for 30min after the dropwise addition is complete. Then 4.35 g (40 mmol) of TMS-Cl was slowly added dropwise, and the temperature was raised to 0° C. for 4 h. After completion, add ice water to quench. DCM (80mL*2) was added for extraction. The collected organic phase was rotary evaporated to obtain a pale yellow oil. Crystallization using Tol / EtOH afforded a pale yellow solid. 6.45 g (15 mmol) of light yellow solid, anhydrous toluene solution (70 mL) and 0.76 mL (8 mmol) of boron tribromide were successively added into a 200 mL stuffy jar....
Embodiment 3
[0121] Synthesis of Compound M3
[0122]
[0123] The specific synthesis steps of compound M3 are as follows.
[0124]
[0125] In a 200ml three-necked flask, 8.84g (20mmol) of substrate A (20mmol) and THF (80mL) were added to dissolve, and nitrogen replacement was performed three times. Cool down to -78°C, and when the temperature reaches, control the temperature below -65°C and slowly add n-BuLi 20mL (50mmol) dropwise, and stir for 30min after the dropwise addition is complete. Then 4.35 g (40 mmol) of TMS-Cl was slowly added dropwise, and the temperature was raised to 0° C. for 4 h. After completion, add ice water to quench. DCM (80mL*2) was added for extraction. The collected organic phase was rotary evaporated to obtain a pale yellow oil. Crystallization using Tol / EtOH afforded a pale yellow solid. 6.45 g (15 mmol) of light yellow solid, anhydrous toluene solution (70 mL) and 0.76 mL (8 mmol) of boron tribromide were successively added into a 200 mL stuffy jar....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com