Pyrazolopyrimidinone compound and preparation method and application thereof
A technology of pyrazolopyrimidinone and azolopyrimidinone, which is applied in the field of pyrazolopyrimidinone compounds and their preparation, can solve problems such as limitations in the use range, achieve good inhibitory effect, excellent inhibitory activity, and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
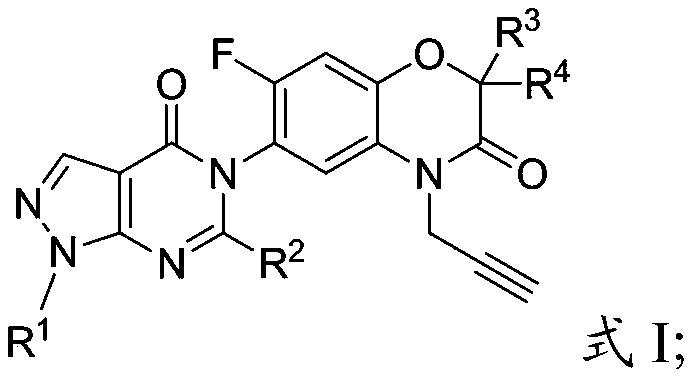
[0050] The present invention provides a preparation method for the pyrazolopyrimidinone compound described in the above technical scheme, comprising the following steps:
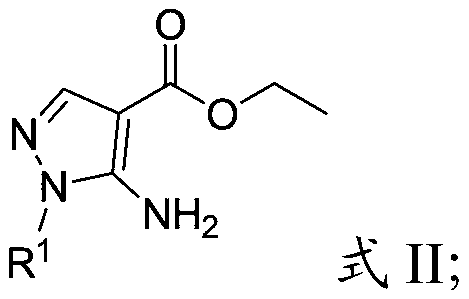
[0051] Will R 1 substituted 5-amino-1H-pyrazole-4-carboxylic acid ethyl ester, tetrahydrofuran, methanol and sodium hydroxide solution are mixed, and hydrolysis is carried out to obtain the first intermediate;
[0052] mixing the first intermediate with thionyl chloride to perform a first substitution reaction to obtain a second intermediate;
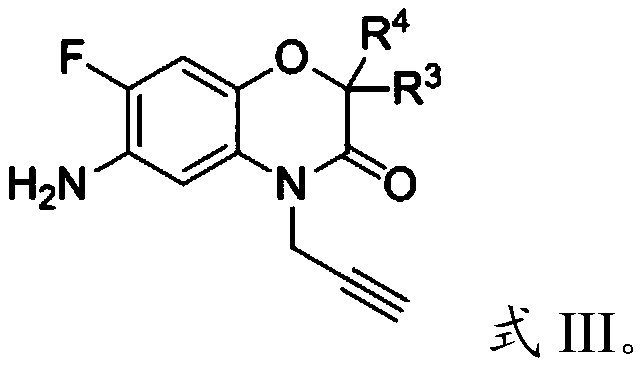
[0053] The second intermediate, dichloromethane, pyridine and R 3 , R 4 Substituted 4-propargyl-6-amino-7-fluoro-2H-benzo[b][1,4]oxazin-3(4H)-ones are mixed and subjected to a second substitution reaction to obtain a third intermediate ;
[0054] Mix the third intermediate, triethoxy compound and acetic anhydride for condensation-elimination reaction to obtain pyrazolopyrimidinone compounds; the triethoxy compound is triethyl orthoformate or triethyl orthoacetate ...
Embodiment 1
[0067]Mix 2.01g of 1-methyl-5-amino-1H-pyrazole-4-carboxylic acid ethyl ester, 4mL of tetrahydrofuran, 12mL of anhydrous methanol and 6mL of 3mol / L sodium hydroxide aqueous solution, reflux at 60°C for 4h, and carry out Hydrolysis reaction; after the reaction is completed, the resulting product system is subjected to vacuum distillation to remove most of the solvent, and then the resulting residue is acidified to pH=1 with 6mol / L hydrochloric acid, and a milky white precipitate appears, and the resulting precipitate system is filtered, washed, and Dichloromethane was washed and dried to obtain the first intermediate (1-methyl-5-amino-1H-pyrazole-4-carboxylic acid, 1.59 g), with a yield of 95%;
[0068] Add 0.47g of the first intermediate to the round bottom flask, then cool in an ice bath to control the temperature to 0°C, add 10mL of thionyl chloride dropwise with a dropping funnel to obtain a clear solution, after the dropwise addition, the resulting system is naturally raise...
Embodiment 2
[0073] Mix 2.01g of 1-methyl-5-amino-1H-pyrazole-4-carboxylic acid ethyl ester, 4mL of tetrahydrofuran, 12mL of anhydrous methanol and 6mL of 3mol / L sodium hydroxide aqueous solution, reflux at 60°C for 4h, and carry out Hydrolysis reaction; after the reaction is completed, the resulting product system is subjected to vacuum distillation to remove most of the solvent, and then the resulting residue is acidified to pH=1 with 6mol / L hydrochloric acid, and a milky white precipitate appears, and the resulting precipitate system is filtered, washed, and Dichloromethane was washed and dried to obtain the first intermediate (1-methyl-5-amino-1H-pyrazole-4-carboxylic acid, 1.59 g), with a yield of 95%;
[0074] Add 0.47g of the first intermediate to the round-bottomed flask, then cool in an ice bath to control the temperature to 0°C, add 10mL of thionyl chloride dropwise with a dropping funnel to obtain a clear solution, after the dropwise addition, the resulting system is naturally ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com