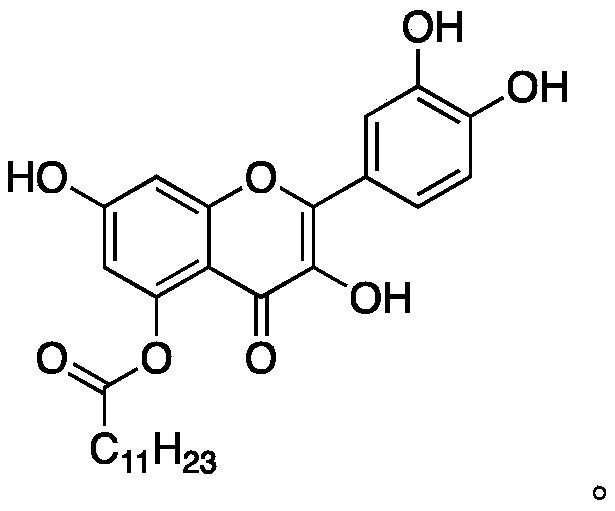
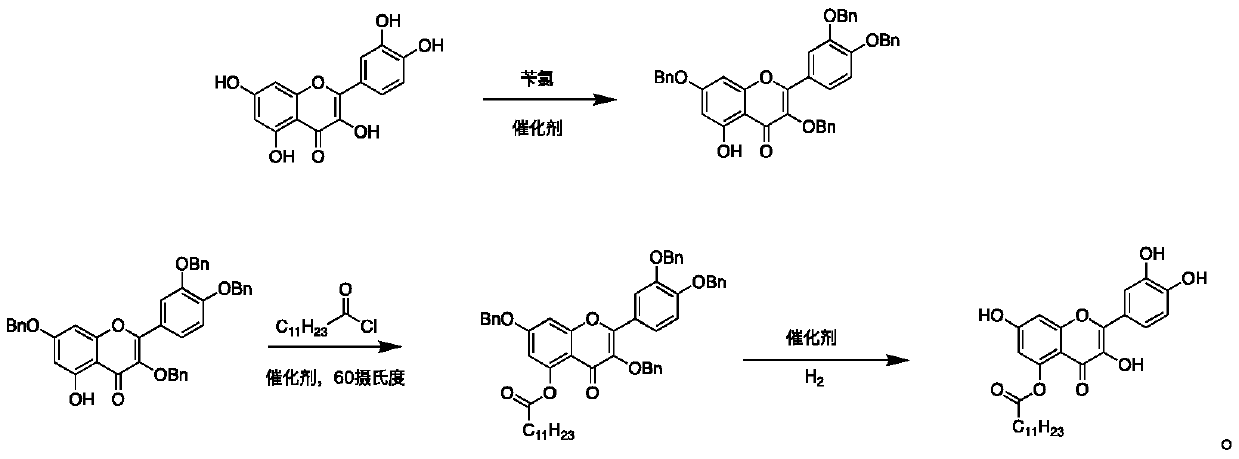
Quercetin derivative and preparation method thereof
A technology of quercetin and its derivatives, which is applied in the field of quercetin derivatives and their preparation, can solve the problems of poor water solubility and low bioavailability of quercetin, achieve simple synthesis methods, improve bioavailability, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of preparation method of quercetin derivative comprises the steps:
[0035] Step 1: Preparation of Compound A
[0036] Add 20 mL of DMF to a 100 mL three-neck flask, then add 604 mg of quercetin, add 1308 mg of potassium carbonate and 550 μL of benzyl chloride to the reaction solution, protect the whole process with nitrogen, stir at room temperature for 8 hours, add 30 mL of ethyl acetate and 10 mL of pure water, extraction, liquid separation, and the aqueous layer was extracted twice with 30mL ethyl acetate, and the organic layer obtained from the combined extraction was suction filtered, spin-dried, and purified by 200-300 mesh silica gel column chromatography to obtain compound A, namely 3,7- Bis(benzyloxy)-2-(3,4-bis(benzyloxy)phenyl)-5-hydroxy-4H-pyran-4-one;
[0037] Step 2: Preparation of Compound B
[0038] Dissolve 500 mg of compound A in 20 mL of DMF, add 600 μL of lauryl chloride and 300 mg of sodium carbonate to the reaction solution, cover the who...
Embodiment 2
[0042] A kind of preparation method of quercetin derivative comprises the steps:
[0043] Step 1: Preparation of Compound A
[0044] Add 20 mL of DMF to a 100 mL three-neck flask, then add 604 mg of quercetin, add 986 mg of potassium carbonate and 550 μL of benzyl chloride to the reaction solution, protect the whole process with nitrogen, stir at room temperature for 10 hours, add 30 mL of ethyl acetate and 10 mL of pure water, extraction, liquid separation, and the aqueous layer was extracted twice with 30mL ethyl acetate, and the organic layer obtained from the combined extraction was suction filtered, spin-dried, and purified by 200-300 mesh silica gel column chromatography to obtain compound A, namely 3,7- Bis(benzyloxy)-2-(3,4-bis(benzyloxy)phenyl)-5-hydroxy-4H-pyran-4-one;
[0045] Step 2: Preparation of Compound B
[0046] Dissolve 500 mg of compound A in 20 mL of DMF, add 600 μL of lauryl chloride and 300 mg of sodium carbonate to the reaction solution, cover the who...
Embodiment 3
[0050] A kind of preparation method of quercetin derivative comprises the steps:
[0051] Step 1: Preparation of Compound A
[0052] Add 20mL THF to a 100mL three-necked flask, then add 604mg of quercetin, add 580mg of DMAP and 550μL of benzyl chloride to the reaction solution, protect the whole process with nitrogen, stir at room temperature for 10 hours, add 30mL of ethyl acetate and 10ml of purified water, Extraction, liquid separation, the aqueous layer continued to be extracted twice with 30mL ethyl acetate, and the organic layers obtained from the combined extraction were suction filtered, spin-dried, and purified by 200-300 mesh silica gel column chromatography to obtain compound A, namely 3,7-bis( Benzyloxy)-2-(3,4-bis(benzyloxy)phenyl)-5-hydroxy-4H-pyran-4-one;
[0053] Step 2: Preparation of Compound B
[0054] 500 mg of Compound A was dissolved in 20 mL of THF, 600 μL of lauryl chloride and 300 mg of DMAP were added to the reaction solution, under nitrogen protect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com