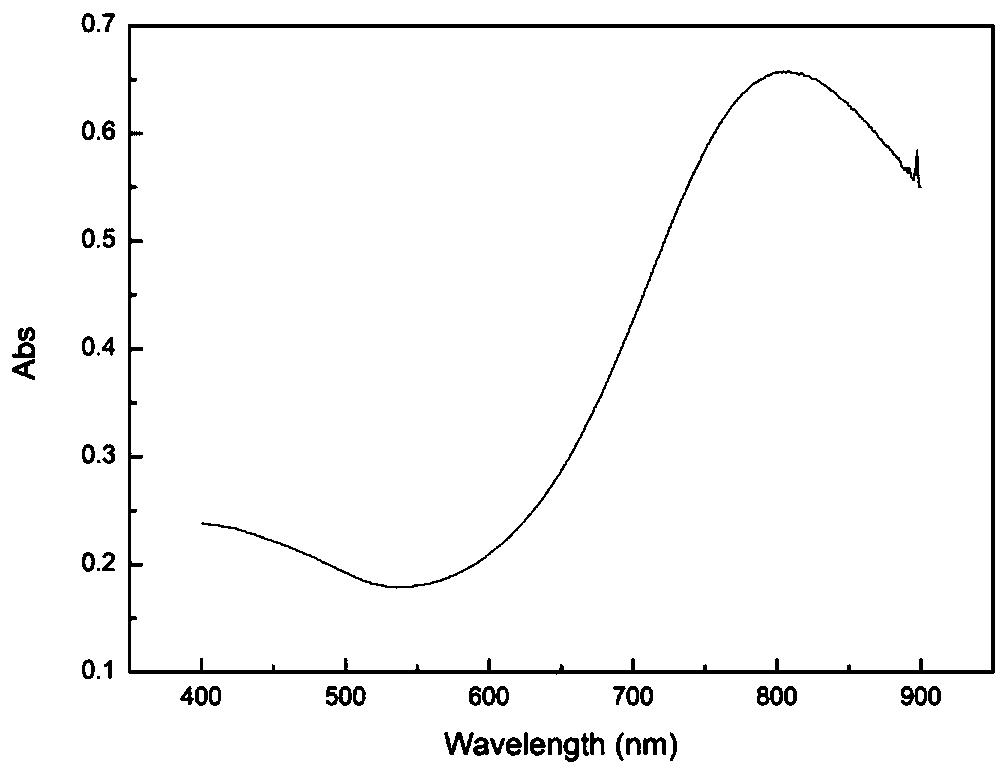
Near-infrared responsive pramipexole-loaded degradable microsphere preparation
A near-infrared and microsphere technology, applied in the field of medicine, can solve the problems of inconvenient and rapid drug delivery, and achieve good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Dissolve 0.12mM cobalt chloride, 0.1mM sodium citrate, and 0.4mM polyvinylpyrrolidone in 200mL deionized water, and dissolve under magnetic stirring;
[0033] 2) Quickly add 0.1 mM sodium borohydride solution to the solution in step 1) under the protection of argon, and stir magnetically for 1 h;
[0034] 3) Add 0.0012mM chloroauric acid to the solution obtained in step 2), and stir magnetically for 2 hours;
[0035] 4) Centrifuge and wash the solution obtained in step 3) for 3 times, collect HGNS, and resuspend HGNS with 0.15% polyvinylpyrrolidone solution, quickly cool down the HGNS suspension to 4°C in the dark, and use power 4W, Ultrasonic treatment for 1 min, freeze-drying;
[0036] 5) Weigh 20mg of pramipexole and dissolve in dimethyl sulfoxide, weigh 200mg of PLGA and dissolve in dichloromethane, ultrasonically dissolve, and combine as the organic phase; weigh 1mg of HGNS protected by polyvinylpyrrolidone obtained in step 4) As a solid phase, colostrum is fo...
Embodiment 2
[0039] 1) Dissolve 0.12mM cobalt chloride, 0.1mM sodium citrate, and 0.4mM polyvinylpyrrolidone in 200mL deionized water, and dissolve under magnetic stirring;
[0040] 2) Quickly add 0.5mM sodium borohydride solution to the solution in step 1) under the protection of argon, and stir magnetically for 1 hour;
[0041] 3) Add 0.0012mM chloroauric acid to the solution obtained in step 2), and stir magnetically for 2 hours;
[0042] 4) Centrifuge and wash the solution obtained in step 3) for 3 times, collect HGNS, and resuspend HGNS with 0.15% polyvinylpyrrolidone solution, quickly cool down the HGNS suspension to 4°C in the dark, and use power 4W, Ultrasonic treatment for 1 min, freeze-drying;
[0043] 5) Weigh 20mg of pramipexole and dissolve in dimethyl sulfoxide, weigh 200mg of PLGA and dissolve in dichloromethane, ultrasonically dissolve, and combine as the organic phase; weigh 1mg of HGNS protected by polyvinylpyrrolidone obtained in step 4) As a solid phase, colostrum is ...
Embodiment 3
[0046] 1) Dissolve 0.12mM cobalt chloride, 0.1mM sodium citrate, and 0.4mM polyvinylpyrrolidone in 200mL deionized water, and dissolve under magnetic stirring;
[0047]2) Quickly add 0.1 mM sodium borohydride solution to the solution in step 1) under the protection of argon, and stir magnetically for 1 h;
[0048] 3) Add 0.0012mM chloroauric acid to the solution obtained in step 2), and stir magnetically for 2 hours;
[0049] 4) Centrifuge and wash the solution obtained in step 3) for 3 times, collect HGNS, and resuspend HGNS with 0.15% polyvinylpyrrolidone solution, quickly cool down the HGNS suspension to 4°C in the dark, and use power 4W, Ultrasonic treatment for 1 min, freeze-drying;
[0050] 5) Weigh 40mg of pramipexole and dissolve in dimethyl sulfoxide, weigh 200mg of PLGA and dissolve in dichloromethane, ultrasonically dissolve, and combine as the organic phase; weigh 1mg of HGNS protected by polyvinylpyrrolidone obtained in step 4) As a solid phase, colostrum is for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com