Injectable celecoxib formulations based on microspheres
A technology of celecoxib and microspheres, applied in the field of injectable celecoxib preparations based on microspheres
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0151] The present invention addresses an unmet need in the art by providing a surprisingly superior way of using celecoxib to treat joint-related diseases. The present invention does this by releasing celecoxib over time from celecoxib-bearing microspheres.
[0152] Specifically, the present invention provides biodegradable microspheres, wherein the microspheres (i) have a diameter of 1 μm to 500 μm; (ii) comprise a polylactic-co-glycolic acid (PLGA) matrix; (iii) carry the drug celecoxib and (iv) release celecoxib for at least one month when present in suitable joint-related tissues.
[0153] In one embodiment of the biodegradable microspheres of the present invention, they have a molar ratio of lactic acid to glycolic acid of 100:0 to 50:50. Preferably, the microspheres of the present invention (i) have a diameter of 20 μm to 200 μm; and (ii) have a molar ratio of lactic acid to glycolic acid of 75:25.
[0154] In another embodiment of the biodegradable microspheres of th...
Embodiment 1
[0180] Example 1. General Experimental Materials and Methods
[0181] PLGA refers to polylactic acid-glycolic acid copolymer; PDLA refers to poly-D-lactic acid, which is a type of polylactic acid (PLA); DMSO refers to dimethyl sulfoxide; PVA refers to polyvinyl alcohol (molecular weight: 31K to 50K, 98% to 99% hydrolyzed); PBS refers to phosphate buffered saline (pH 7.4); and MW refers to molecular weight. The magnetic stir bar used in all the following examples has a length of 2 cm and a diameter of 0.7 cm.
[0182] (i) PDLA (0.4 dl / g, ester-terminated): intrinsic viscosity = 0.35 dl / g to 0.45 dl / g. MW: 20,000 to 30,000; (ii) PDLA (0.6dl / g, ester-terminated): intrinsic viscosity = 0.55dl / g to 0.65dl / g; (iii) PDLA (2dl / g, ester-terminated): intrinsic viscosity = 1.6 dl / g to 2.4 dl / g. MW: 300,000 to 600,000; (iv) PLGA (50:50, 0.2 dl / g, acid-terminated): intrinsic viscosity = 0.16 dl / g to 0.24 dl / g. MW: 7,000 to 17,000; (v) PLGA (50:50, 0.2 dl / g, ester-terminated): intrins...
Embodiment 2
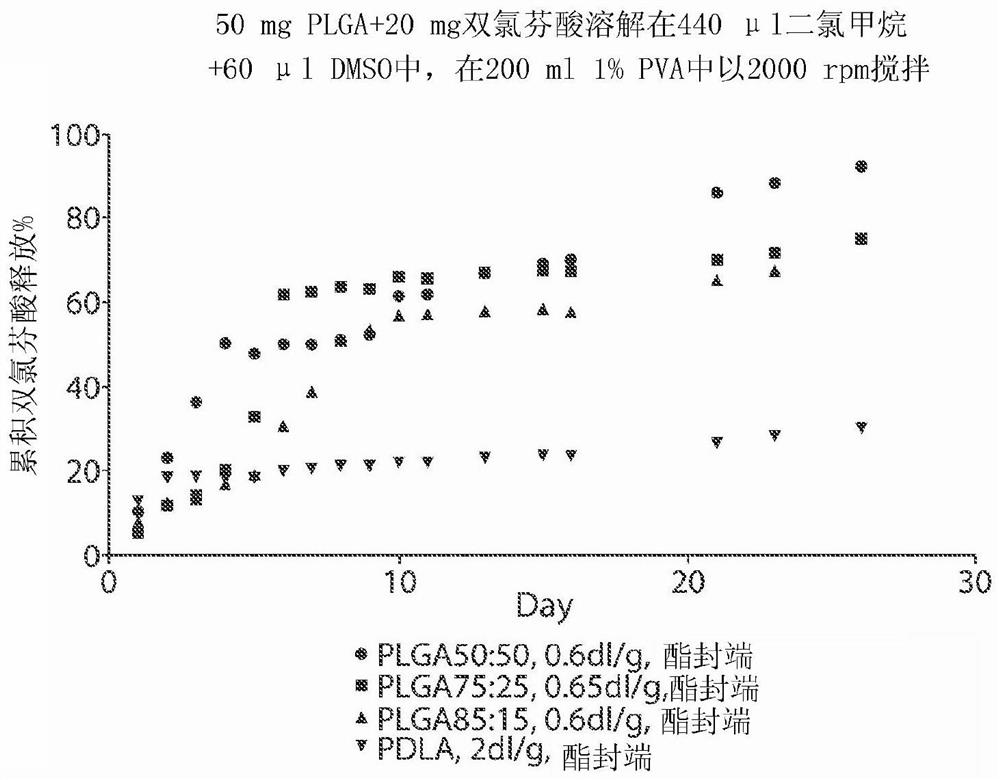
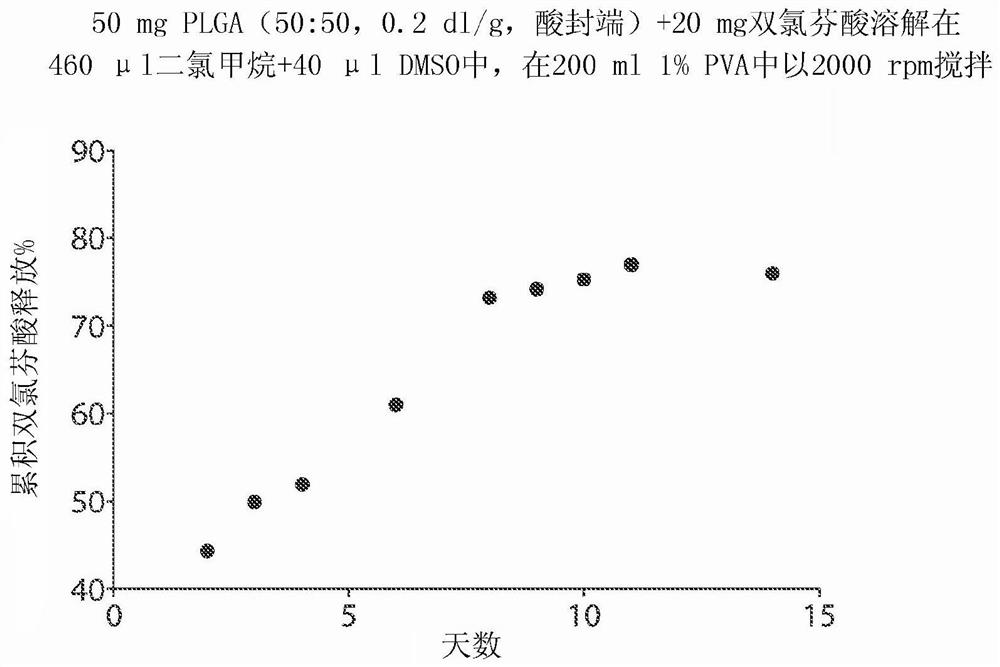
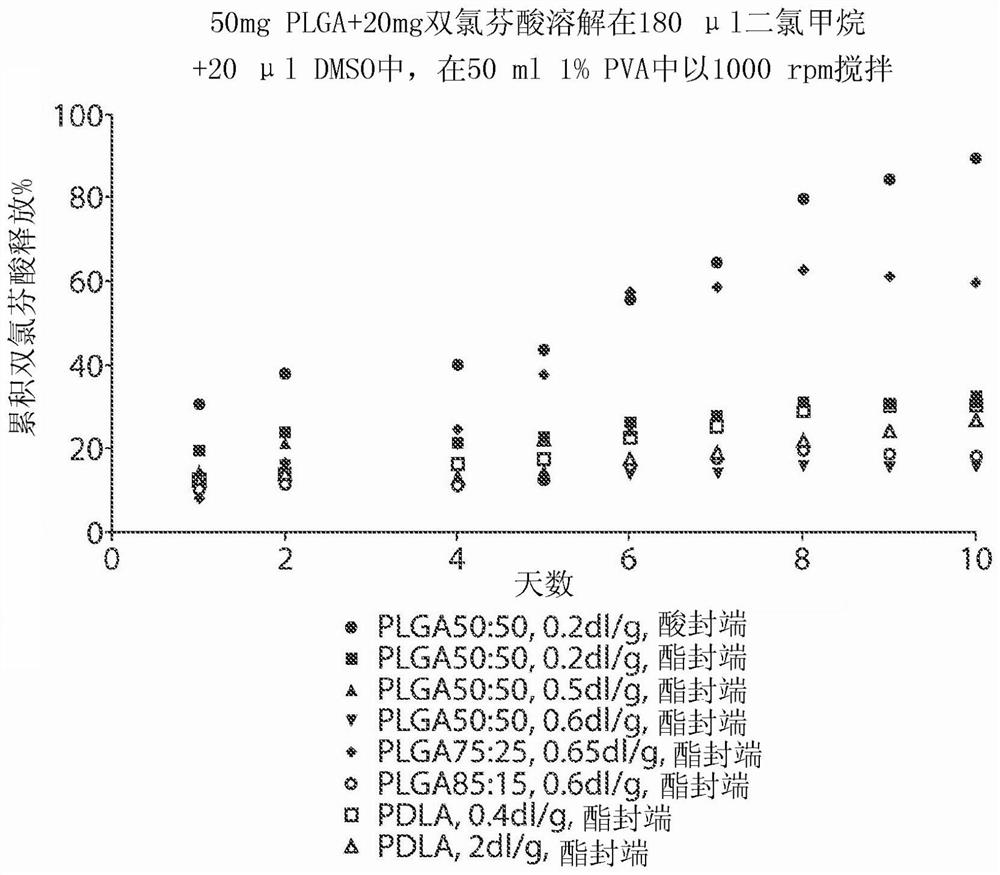
[0184] Example 2. Microsphere Formulation of Diclofenac: Effect of Polymer Composition on Release of Diclofenac (I)
[0185] A drug depot comprising NSAID, diclofenac (2-[2-(2,6-dichloroaniline)phenyl]acetic acid) was prepared and incorporated into microspheres. Formulations were prepared by dissolving 50 mg PLGA or PDLA and 20 mg diclofenac in 440 μl dichloromethane and 60 μl DMSO. 500 μl of the solution was injected into the center of 200 ml of 1% PVA (w / v, pH adjusted to 2) in a 250 ml beaker, stirred with a magnetic stirring bar at 2,000 rpm to form an emulsion. The emulsion was stirred at 2,000 rpm for 2 minutes and at about 500 rpm for 2 hours to evaporate the dichloromethane. Microspheres were harvested by centrifugation at 4,000g for 5 minutes and washed three times with water.
[0186] To test the release of diclofenac from the microspheres, all prepared microspheres were added to 250 ml of 10 mM PBS and shaken at 80 rpm at 37 °C. At each time point in the releas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com