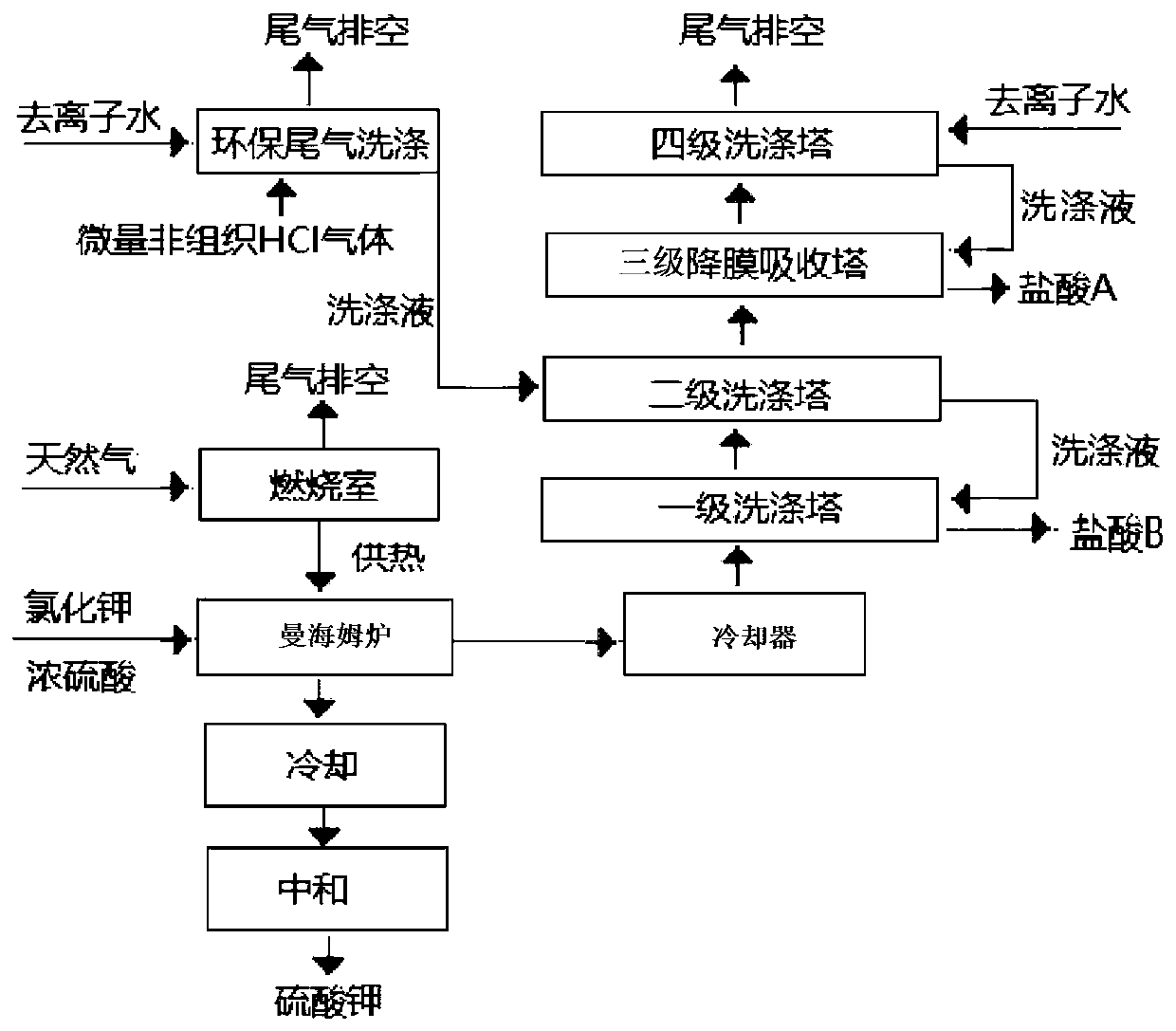
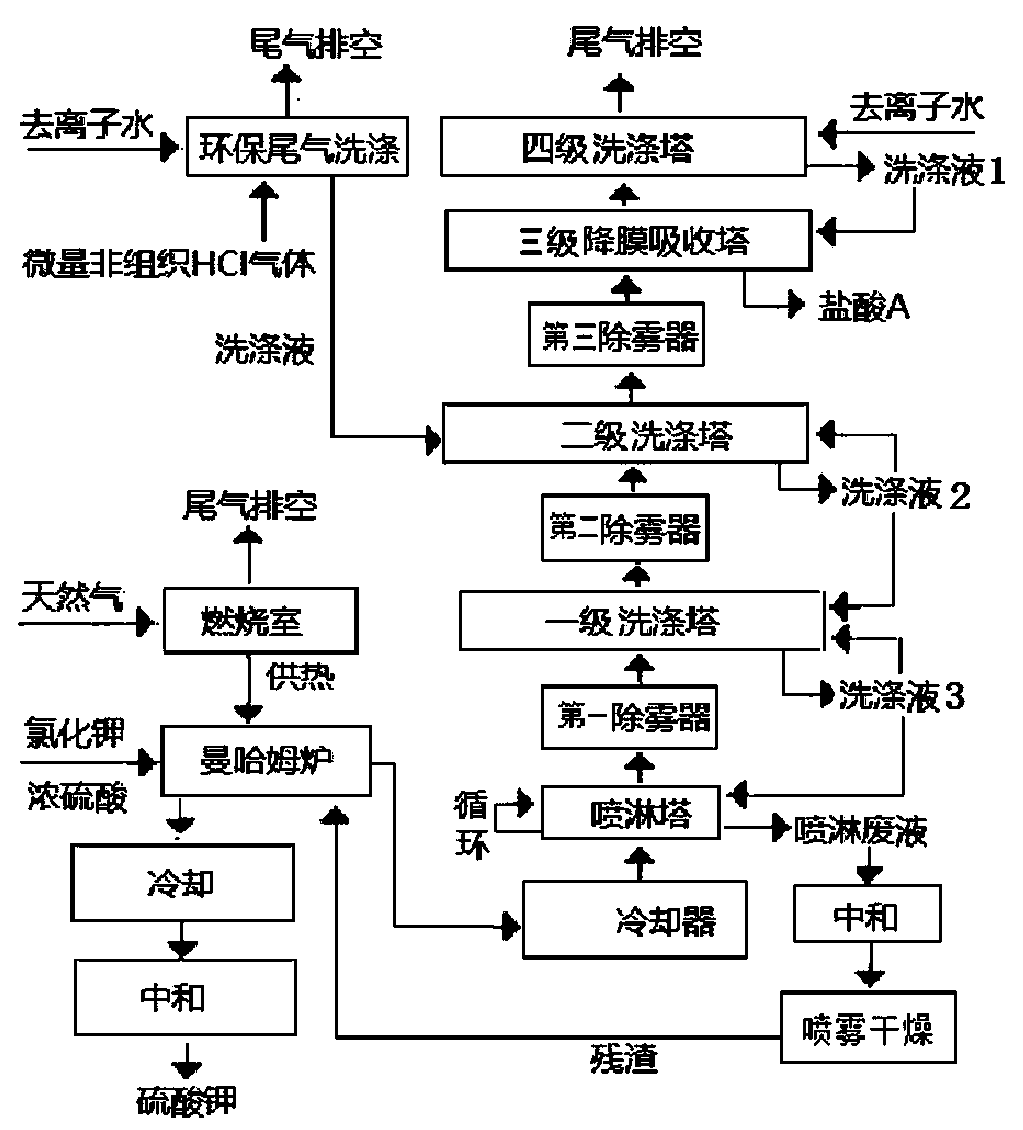
Method for preparing hydrochloric acid by utilizing hydrogen chloride gas in the process of producing potassium sulfate by Mannheim method
A Mannheim process, potassium sulfate technology, applied in chloride preparation, sulfate/bisulfate preparation, chlorine/hydrogen chloride, etc., can solve the problems of low quality, economic loss of enterprises, and high impurity content of hydrochloric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The flow of deionized water for washing the gas in the four-stage scrubber is 14.8L / min, the internal circulation is 100L / min, and the output of hydrochloric acid A is 30.1 tons / day. The flow rate of deionized water for cleaning the environmentally friendly tail gas is 0.7L / min, and the output of mixed solids obtained from spraying waste liquid is 294kg / day.
[0042] The quality of detected hydrochloric acid A is shown in table 3 below:
[0043] project Sulfate (SO 4 2- ,%)
Fe 3+ (%)
HCl(%) color hydrochloric acid A 0.008 0.0005 32.1 colorless
[0044] table 3
[0045] The content of hydrogen chloride in the tail gas is: 5.17mg / m 3 , in line with environmental emission standards.
Embodiment 2
[0047] The flow of deionized water for washing the gas in the four-stage scrubber is 13.2L / min, the internal circulation is 115L / min, and the output of hydrochloric acid A is 28.0 tons / day. The flow rate of deionized water for cleaning the environmentally friendly tail gas is 2.6L / min, and the output of mixed solids obtained from spraying waste liquid is 299kg / day.
[0048] The hydrochloric acid quality after testing is shown in table 4 below:
[0049] project Sulfate (SO 4 2- ,%)
Fe 3+ (%)
HCl(%) color hydrochloric acid A 0.007 0.0005 32.6 colorless
[0050] Table 4
[0051] The content of hydrogen chloride in the tail gas is: 4.93mg / m 3 , in line with environmental emission standards.
Embodiment 3
[0053] The flow of deionized water for washing the gas in the four-stage scrubber is 14.8L / min, the internal circulation is 135L / min, and the output of hydrochloric acid A is 29.3 tons / day. The flow rate of deionized water for cleaning the environmentally friendly tail gas is 3.0L / min, and the output of mixed solids obtained from spraying waste liquid is 301kg / day.
[0054] The quality of detected hydrochloric acid A is shown in Table 5 below:
[0055] project Sulfate (SO42-,%) Fe3+(%) HCl(%) color hydrochloric acid A 0.007 0.0004 32.4 colorless
[0056] table 5
[0057] The content of hydrogen chloride in the tail gas is: 4.98mg / m 3 , in line with environmental emission standards.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com