Imidazoline mannich base corrosion inhibitor and preparation method thereof
A Mannich base-like and imidazoline-like technology is applied in the field of imidazoline-like Mannich base corrosion inhibitor and its preparation, and can solve problems such as wellbore pipe wall corrosion, safety accident, oil pipe fracture and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
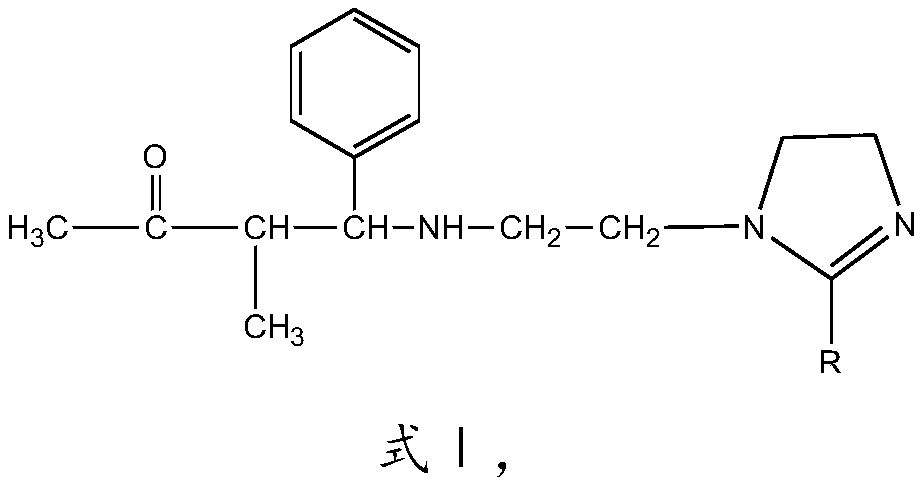
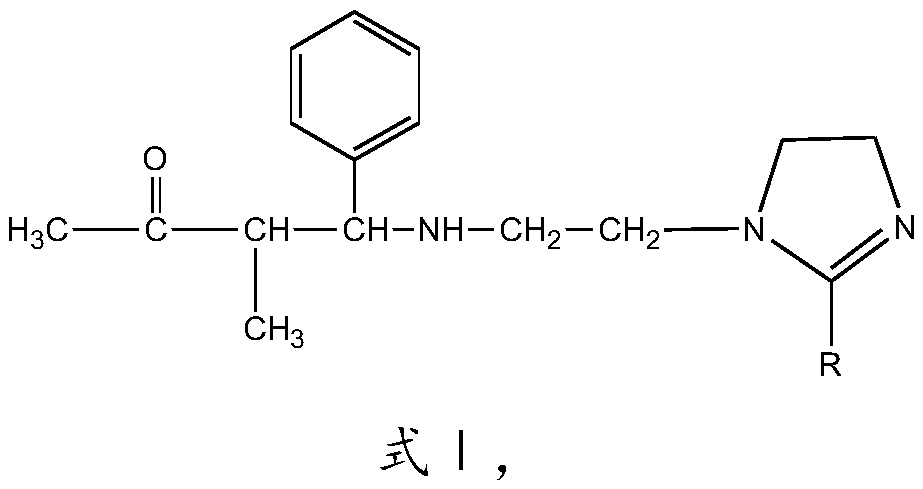
[0028] The imidazoline Mannich base corrosion inhibitor of this embodiment has the structure shown in formula I:
[0029]
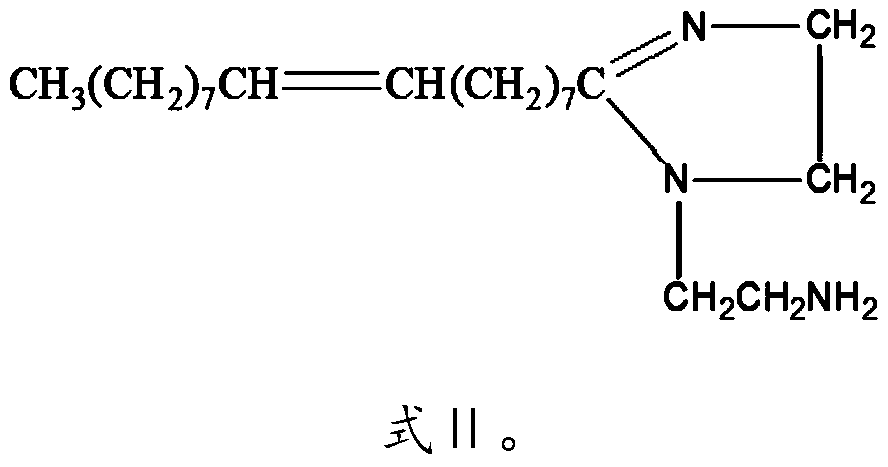
[0030] R is CH 3 (CH 2 ) 7 CH=CH(CH 2 ) 7 —.
[0031] The imidazoline Mannich base corrosion inhibitor of the present embodiment is made from the raw materials in the following mass percentages: 18.1% benzaldehyde, 12.4% methyl ethyl ketone, 47.9% imidazoline oleate, 17.2% ethanol, 4.3% concentrated hydrochloric acid, Catalyst AlCl 3 0.1%.
[0032] Described imidazoline oleate is made by the preparation method comprising the following steps:
[0033] 1) Mix oleic acid and diethylenetriamine at a molar ratio of 1:1.2, heat to 160°C, keep at a constant temperature for 2 hours, and continue to heat up to 180°C for 2 hours to obtain an amide;
[0034] 2) Continue to heat up the amide obtained in step 1) to 210°C, keep the temperature constant for 2 hours, then continue to heat up to 230°C, keep the temperature for 2 hours, stop heating after the p...
Embodiment 2
[0042] The structure of the imidazoline Mannich base corrosion inhibitor in this embodiment is the same as that in Embodiment 1.
[0043] The imidazoline Mannich base corrosion inhibitor of the present embodiment is made from the raw materials in the following mass percentages: 15% benzaldehyde, 10.2% butanone, 49.2% imidazoline oleate, 21.2% ethanol, 4.2% concentrated hydrochloric acid, Catalyst InCl 3 0.2%.
[0044] Described imidazoline oleate is made by the preparation method comprising the following steps:
[0045] 1) Mix oleic acid and diethylenetriamine at a molar ratio of 1:1.2, heat to 150°C, keep at a constant temperature for 2 hours, and continue to heat up to 170°C for 2 hours to obtain an amide;
[0046] 2) Continue to heat up the amide obtained in step 1) to 200°C, keep the temperature for 2 hours, then continue to heat up to 220°C, keep the temperature for 2 hours, stop heating after the produced water meets the requirements, and obtain oleic acid imidazoline...
Embodiment 3
[0051] The structure of the imidazoline Mannich base corrosion inhibitor in this embodiment is the same as that in Embodiment 1.
[0052]The imidazoline Mannich base corrosion inhibitor of the present embodiment is made from the raw materials in the following mass percentages: 12.7% benzaldehyde, 8.6% methyl ethyl ketone, 50.2% imidazoline oleate, 24% ethanol, 4.2% concentrated hydrochloric acid, Catalyst NbCl 5 0.3%.
[0053] Described imidazoline oleate is made by the preparation method comprising the following steps:
[0054] 1) Mix oleic acid and diethylenetriamine at a molar ratio of 1:1.2, heat to 155°C, keep at a constant temperature for 1 hour, and continue to heat up to 175°C for 1 hour to obtain an amide;
[0055] 2) Continue to heat up the amide obtained in step 1) to 205°C, keep the temperature for 2 hours, then continue to heat up to 225°C, keep the temperature for 2 hours, stop heating after the produced water meets the requirements, and obtain oleic acid imid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com