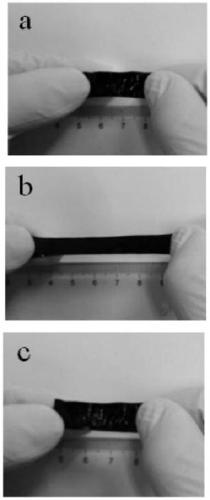
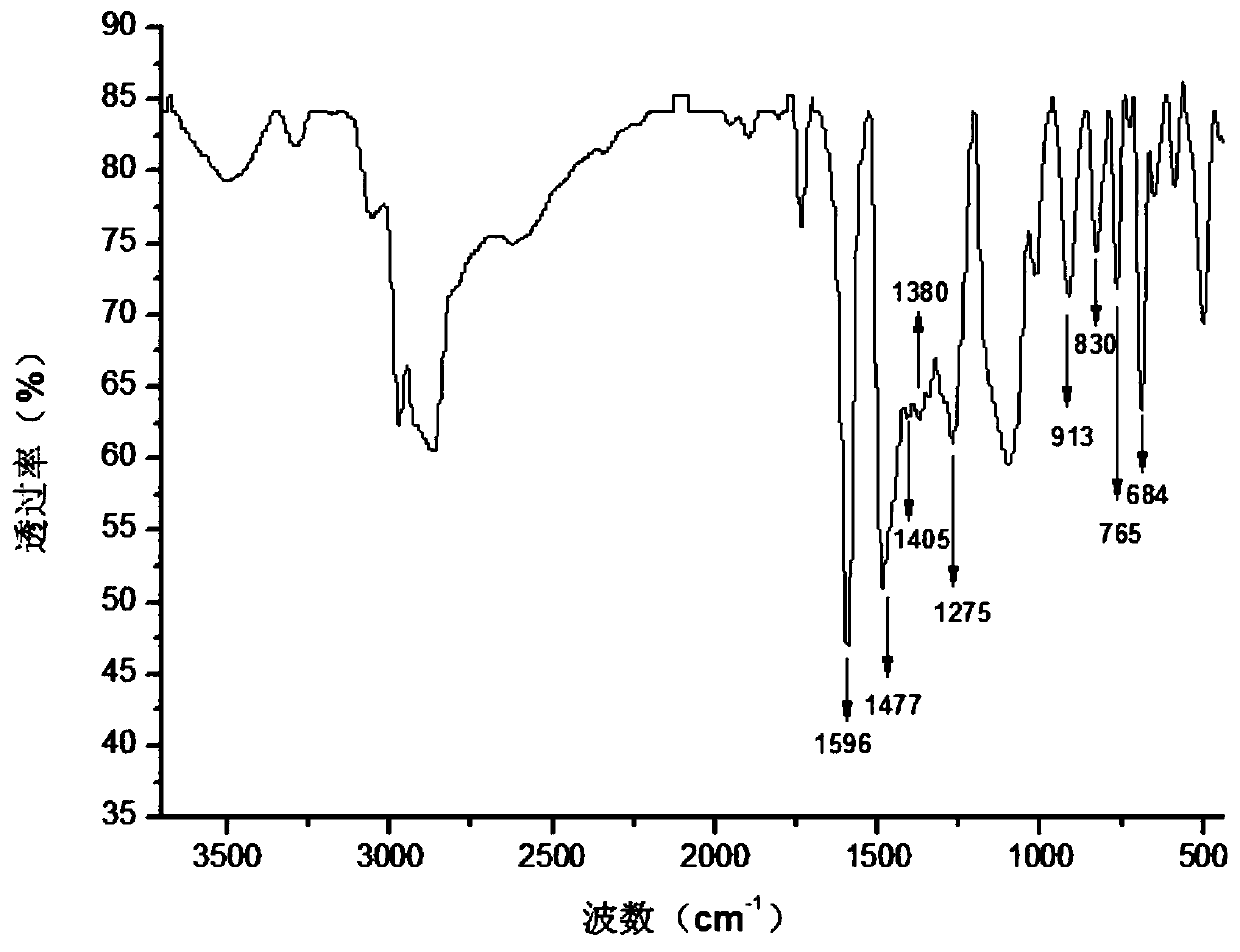
Photoactivity benzoxazine elastomer and preparation method thereof
A benzoxazine and elastomer technology, applied in the field of photoactive benzoxazine elastomer and its preparation, can solve problems such as poor heat resistance, achieve good flexibility and applicability, high resilience, and simple curing process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of photoactive benzoxazine elastomer, the steps are as follows:
[0036] (1) After adding 120 parts by weight of paraformaldehyde and 396 parts by weight of 4-hydroxyazobenzene into the reactor, add 1500 parts by weight of toluene, mechanically stir at 500 rpm for 20 minutes at 25°C, and pour into the system Slowly add 230 parts by weight of polyetheramine D230 dropwise; (2) Reflux the system for 8 hours at 125°C and 500 rpm with mechanical stirring; After 3 times, the organic phase was separated, the toluene was removed by rotary evaporation, and the product was dried to obtain a benzoxazine monomer containing an azo structure; (3) the benzoxazine monomer containing an azo structure was solidified at 180°C After reacting for 2 hours, curing and reacting at 200° C. for 2 hours to prepare an elastomer containing an azo structure x benzoxazine. The reaction process is as follows:
[0037]
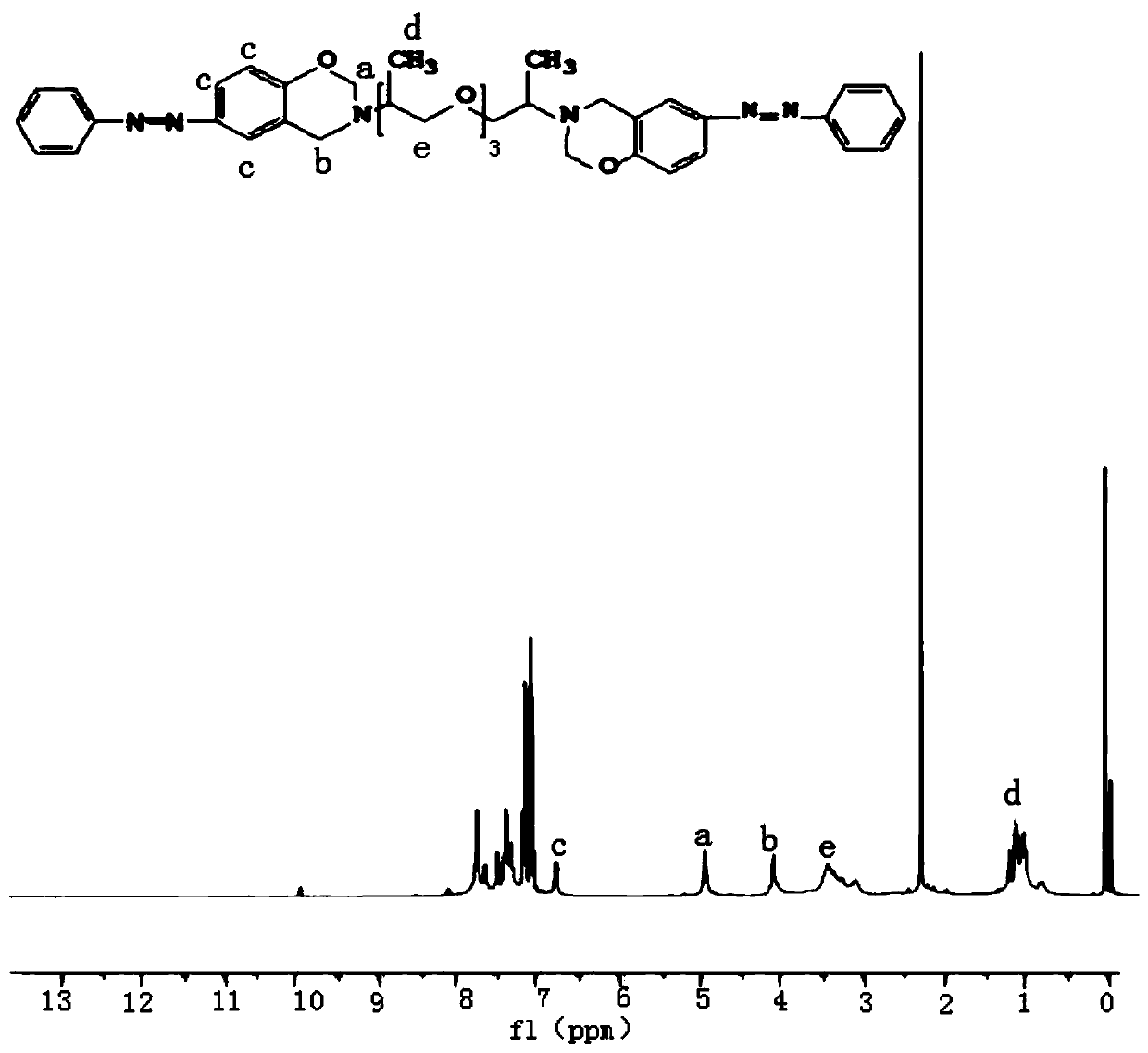
[0038] figure 1It is the NMR characterization diagram o...
Embodiment 2
[0041] A preparation method of photoactive benzoxazine elastomer, the steps are as follows:
[0042] (1) After adding 120 parts by weight of paraformaldehyde and 396 parts by weight of 4-hydroxyazobenzene into the reactor, add 1500 parts by weight of dioxane, and mechanically stir at 500 rpm for 15 minutes at 25°C. Slowly add 400 parts by weight of polyetheramine D400 to the system dropwise; (2) Reflux the system at 125°C and 500 rpm with mechanical stirring for 8 hours. After the reaction is complete, cool the system to room temperature, wash with alkali, After washing with water for 3 times, the organic phase was separated, the dioxane was removed by rotary evaporation, and the product was dried to obtain a benzoxazine monomer containing an azo structure. The azo-structure-containing benzoxazine monomer was cured at 180° C. for 2 hours, and then cured at 200° C. for 2 hours to prepare an azo-structure-containing benzoxazine elastomer. The reaction process is as follows:
...
Embodiment 3
[0048] A preparation method of photoactive benzoxazine elastomer, the steps are as follows:
[0049] (1) After adding 120 parts by weight of paraformaldehyde and 396 parts by weight of 4-hydroxyazobenzene into the reaction kettle, add 1500 parts by weight of ethyl acetate, and mechanically stir at 500 rpm for 10 minutes at 25°C to feed the system Slowly add 2000 parts by weight of polyetheramine D2000 dropwise; (2) Reflux the system at 125°C and 500 rpm with mechanical stirring for 8 hours. After the reaction is completed, cool the system to room temperature and wash with alkali and water. After each 3 times, the organic phase was separated, the ethyl acetate was removed by rotary evaporation, and after the product was dried, a benzoxazine monomer containing an azo structure was obtained; (3) the benzoxazine monomer containing an azo structure was After curing at 180°C for 2 hours, and then curing at 200°C for 2 hours, a benzoxazine elastomer containing an azo structure was pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com