Freeze-drying protective agent for listeria monocytogenes standard substance, freeze-drying preserving method and application
A technology of Listeria monocytogenes and freeze-drying protective agent, which is applied in the direction of microorganism-based methods, biochemical equipment and methods, bacteria, etc., to achieve a high rate of viable bacteria, a simple and feasible freeze-drying preservation method, and a high rate of viable bacteria Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In this embodiment, the resurrection, identification and cultivation of strains are carried out, and the specific operations are as follows:
[0039] The cryopreserved standard Listeria monocytogenes strain was revived by streaking on nutrient agar, and cultured at 37°C for 18h-24h. Pick a single colony of Listeria monocytogenes from the plate and inoculate it into the nutrient broth, culture at 37°C for 18h-24h, take the bacterial liquid and inoculate it on the TSA-YE medium, and perform Gram test at 37°C until the colony grows. Observed under the microscope after staining, the bacteria are arranged in the shape of short rods, with a size of about (0.4μm-0.5μm)×(0.5μm-2.0μm). Randomly pick colonies and use VITEK automatic biochemical analyzer for identification, pick pure culture A single colony was punctured in the SIM power medium, cultured at 30°C for 48 hours, and grew in an umbrella shape above the SIM medium, picked a purely cultured single colony and punctured i...
Embodiment 2
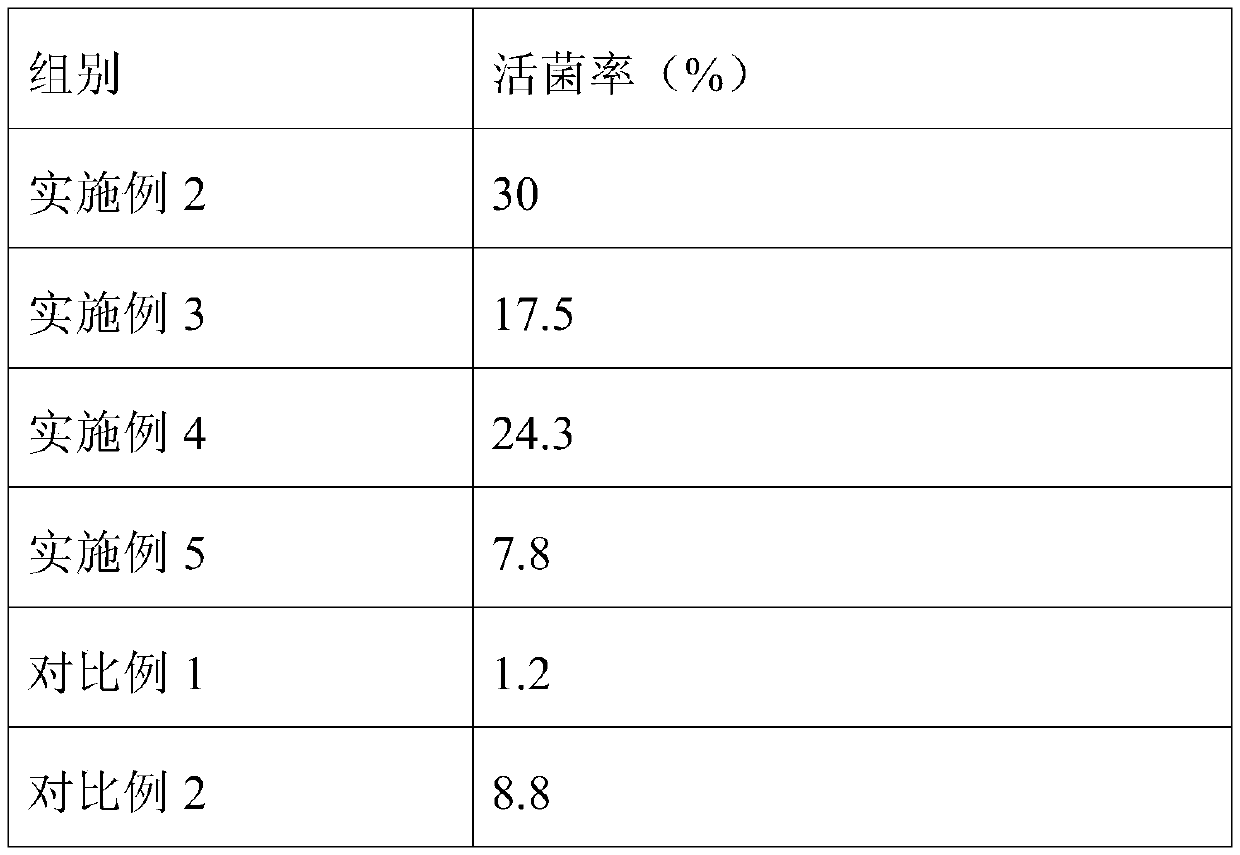
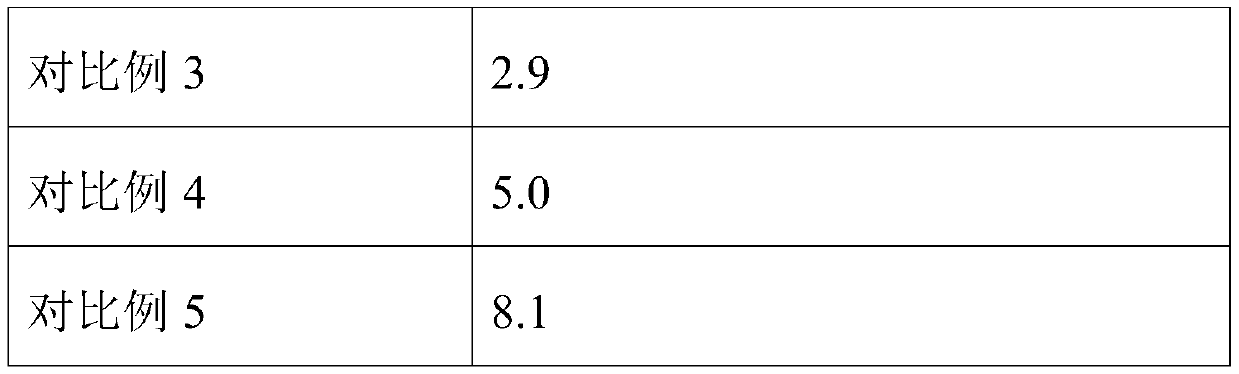
[0041] This embodiment provides a lyoprotectant for reference materials of Listeria monocytogenes. The lyoprotectant includes the following components in terms of mass percentage: 50% skimmed milk, 0.5% polyvinylpyrrolidone, sucrose 4%, tyrosine 0.5%, and the solvent is sterile distilled water.
[0042] Using this lyoprotectant to lyophilize the Listeria monocytogenes reference material comprises the following steps:
[0043] The protective agent was subjected to high-pressure steam sterilization, and then the cell density was 1×10 8 The CFU / mL Listeria monocytogenes suspension and the protective agent were mixed at a volume ratio of 1:100 to obtain a mixture, and then the mixture was distributed into brown vials with a sterile pipette, each bottle 1mL, in- Pre-freezing at 80° C. for 8 hours, freeze-drying at a vacuum degree of 0.015 mbar for 36 hours to obtain a freeze-dried product, and vacuum-capping the freeze-dried standard substance.
Embodiment 3
[0045] This embodiment provides a lyoprotectant for reference material of Listeria monocytogenes, the lyoprotectant comprises the following components in terms of mass percentage: 40% skimmed milk, 0.2% polyvinylpyrrolidone, sucrose 6%, tyrosine 0.8%, and the solvent is sterile distilled water.
[0046] The standard material of Listeria monocytogenes was freeze-dried by using the lyoprotectant, and the operation steps were consistent with those in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com