Novel target for preventing and treating hepatitis C and CRISPR-Cas9 targeting system and application thereof
A viral hepatitis and target technology, applied in the field of biomedicine, can solve the problems of high reinfection rate of hepatitis C patients, drug side effects, high cost, etc., to reduce the defects of incomplete silencing or inability to silence gene expression, high efficiency and specificity Effects of knockout and improved targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 sgRNA design
[0035] Design and synthesis of sgRNA sequence:
[0036] sgRNA was designed for the miR-615-3p gene precursor sequence, and a total of 8 sgRNAs were designed:
[0037] (1) ctcgggaggggcgggaggg
[0038] (2) aggggcgggaggggggtccc
[0039] (3) ggaagaggggagaccccaggct
[0040] (4) ccccggtgctcggatctcga
[0041] (5) tctcgagggtgcttattgtt
[0042] (6) ttattgttcggtccgagcct
[0043] (7) tgggggggaagaggggagaccc
[0044] (8) ggaagaggggagaccccaggct
[0045] After optimized screening, item (8) was selected.
Embodiment 2
[0046] Example 2 Constructing the sgRNA expression vector of miR-615-3p gene
[0047] 1. Preparation of sgRNA sequence DNA fragments
[0048] (1) Synthesize the forward and reverse phosphorylated oligonucleotide sequences of the above-mentioned embodiment 1 (8) sgRNA:
[0049] F(5'-3') (SEQ ID NO.3): CACCG ggaagaggggagaccccaggct
[0050] R(5'-3') (SEQ ID NO.4): AAACagcctgggtctccctcttccC
[0051] The corresponding forward and reverse oligonucleotide sequences are annealed and annealed to form double-stranded DNA fragments with cohesive ends.
[0052] The reaction (10 μL) system is as follows:
[0053]
[0054] (2) Put the above reaction system into the PCR machine, and carry out the reaction according to the following procedure.
[0055] Reaction procedure:
[0056] 95℃ 5min
[0057] 95-25℃ 5℃ / min
[0058] The reaction product was stored at -20°C.
[0059] 2. Construction of sgRNA expression vector
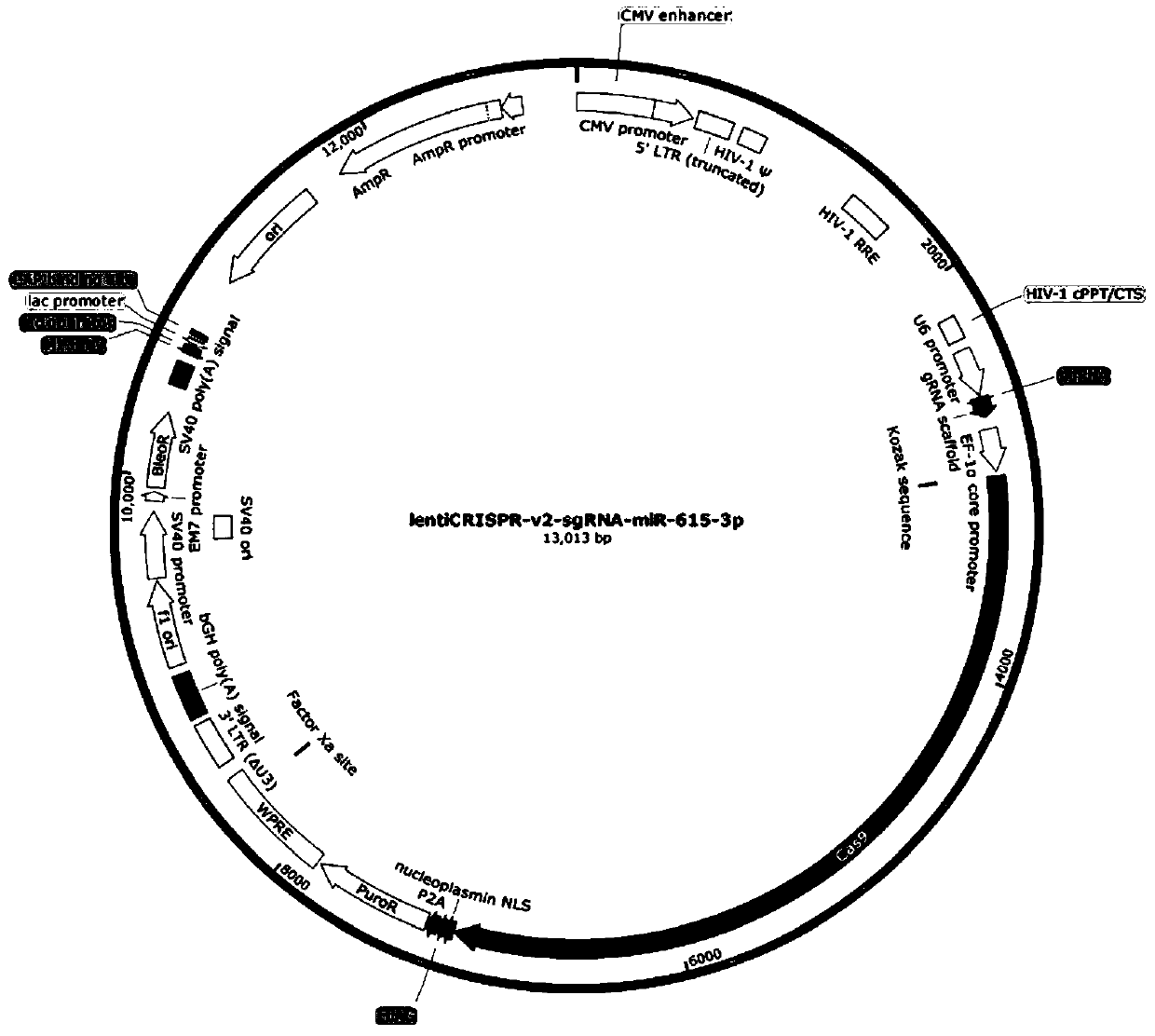
[0060] (1) Digest the target vector lentiCRISPR-v2 plasmid with BsmB...
Embodiment 3
[0070] Example 3 Construction of Huh.7.5 Stable Cell Line by Lentiviral System
[0071] 1. Virus packaging
[0072] By culturing 293FT cells, transfect according to the ratio of core plasmid: packaging plasmid: envelope plasmid = 4:3:2, the core plasmid of the sgRNA group is: lentiCRISPR-v2-sgRNA-miR-615-3p, with lentiCRISPR-v2 as control group. The virus liquid was collected at 24h, 48h and 72h after transfection, and the three virus liquids collected from the cells were mixed and concentrated by ultrafiltration and the virus titer was determined.
[0073] 2. Cell transfection
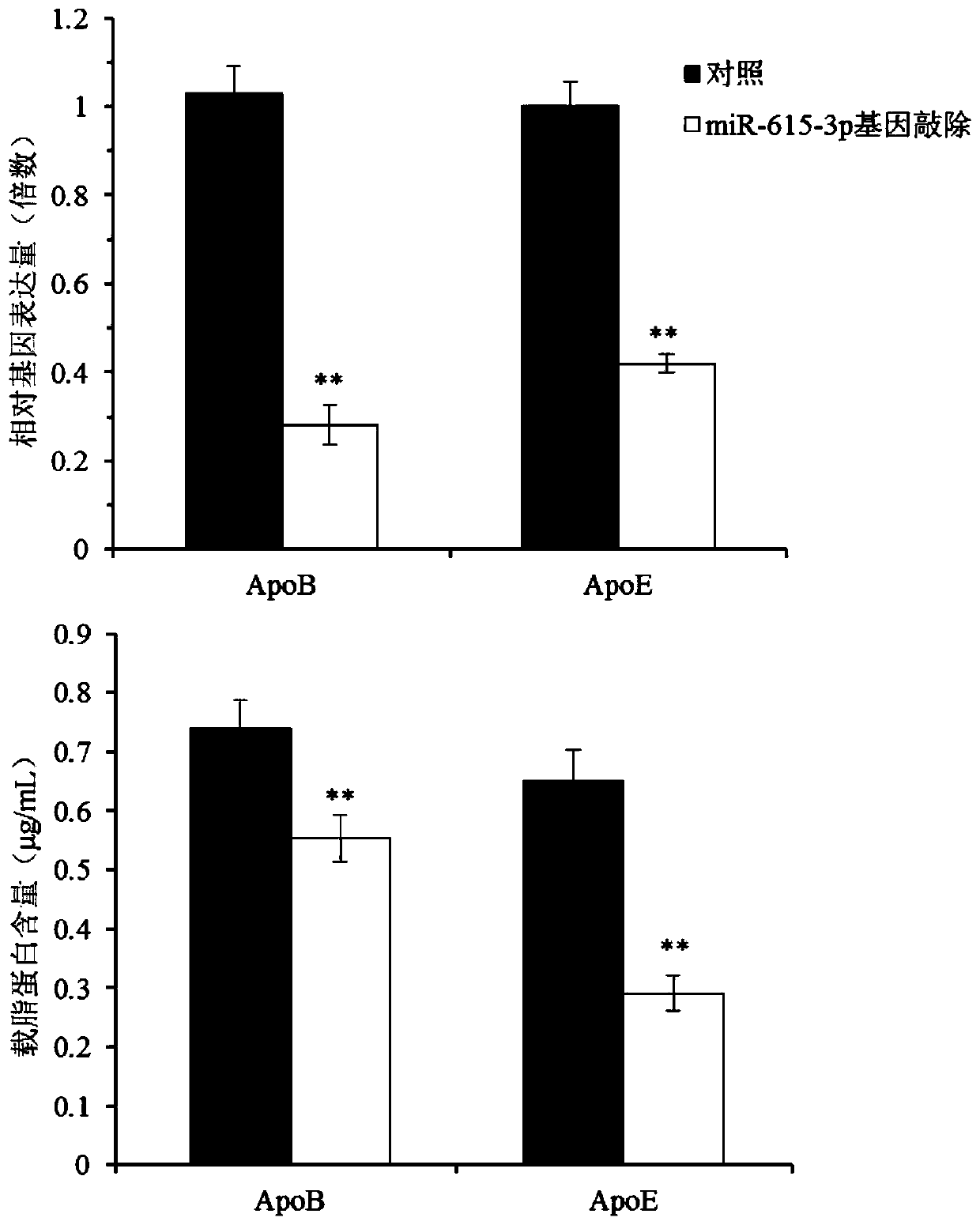
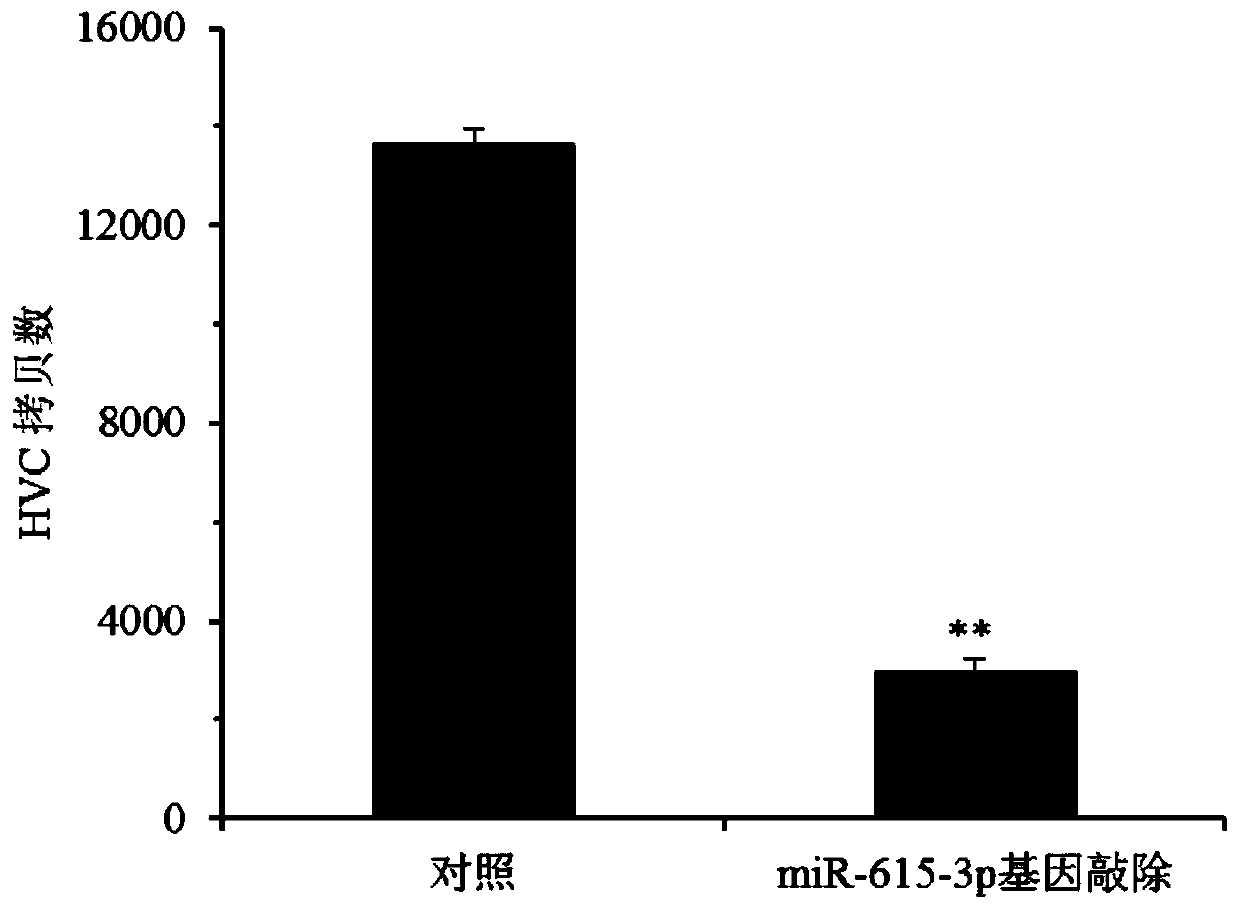
[0074] The Huh.7.5 cells were plated one day in advance, and the cell density reached 40-60% at the time of transfection. The culture medium was removed before transfection, the virus concentrate and transfection reagent were diluted with fresh medium to treat the cells, and 1.0 μg / mL puromycin was used for selection after 96 hours of infection, and the selection time was 7 days. After selection b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com