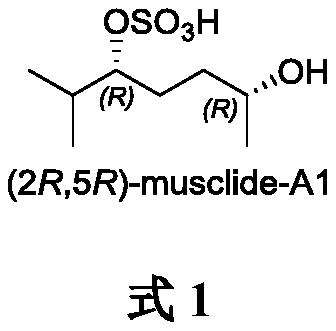
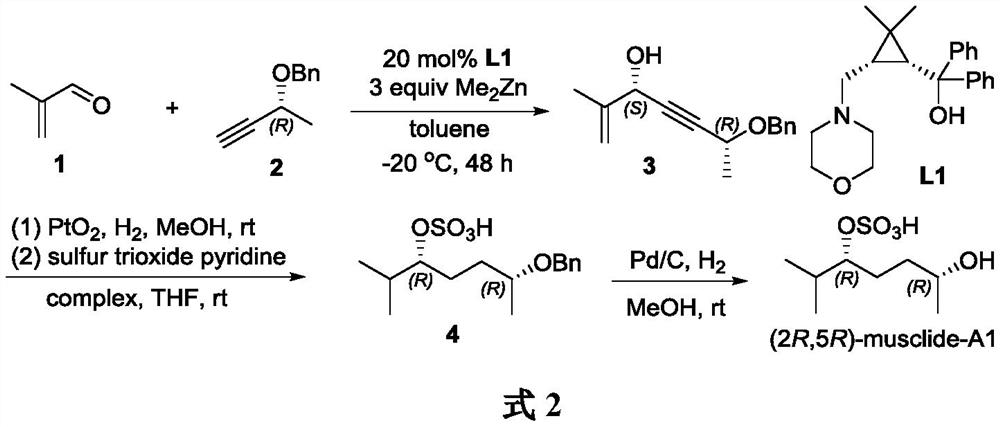
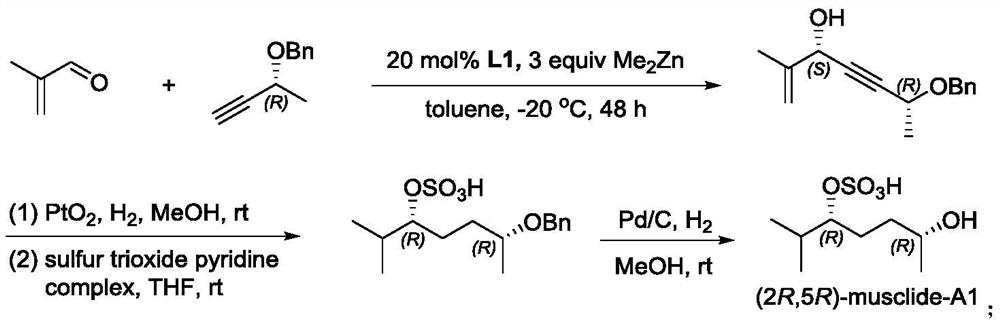
A method for synthesizing musk extract (2r, 5r)-musclide-a1
A synthetic method and technology for synthesizing musk, applied in the preparation of organic compounds, organic chemical methods, chemical instruments and methods, etc., can solve problems such as limitations, low content, lengthy reaction routes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis of (2R,5S)-6-methyl-2-benzyloxy-6-hepten-3-yn-5-ol 3
[0019] Under argon protection, add cyclopropaneaminoalcohol chiral ligand L1 (70.2mg, 0.2mmol, 0.2equiv) into a 10mL Shrek tube, then add toluene (4mL) and (R)-3-benzyloxy-1 - Butyne 2 (480.6mg, 3.0mmol), stir well at room temperature. The mixture was cooled to 0° C., dimethyl zinc (2.5 mL, 1.2M solution in toluene, 1.5 mmol, 3 equiv) was slowly added dropwise, and stirring was continued at this temperature for 2 h. Then the temperature was lowered to -20°C, methacrolein 1 (70.1mg, 1.0mmol) was slowly added dropwise, and the reaction was stirred at -20°C for 48h. After the reaction was completed, the reaction was quenched by adding ice, and the aqueous phase was washed with Et 2 O (3×15 mL) was extracted, and the organic phases were combined. Dry over anhydrous sodium sulfate, concentrate under reduced pressure, and finally purify by silica gel column chromatography (n-hexane / ethyl acetate=5:1) to obtai...
Embodiment 2
[0021] Synthesis of (2R,5R)-6-methyl-2-benzyloxy-5-heptanol sulfate 4
[0022] At 0°C, the Pt 2 O (50.0mg, 0.22mmol) was added to a 25mL Shrek bottle, methanol (2mL) was added under a hydrogen atmosphere, compound 3 (506.0mg, 2.2mmol) was dissolved in methanol (10mL) and added to the reaction system, the reaction temperature rose to room temperature, The reaction was stirred for 8h. After the reaction was completed, it was filtered, and the filtrate was concentrated under reduced pressure to obtain the crude product of 6-methyl-2-benzyloxy-5-heptanol.
[0023] The above crude product was dissolved in THF (10 mL), sulfur trioxide pyridine complex (880.0 mg, 5.5 mmol) was added at 0°C, and the reaction was stirred at room temperature for 2 h. After the reaction was completed, the reaction mixture was concentrated under reduced pressure to obtain a crude product. The crude product was purified by silica gel column chromatography (dichloromethane / methanol=10:1) to obtain (2R,5R...
Embodiment 3
[0025] Synthesis of (2R,5R)-Musclide-A1
[0026] Under hydrogen atmosphere, Pd / C (20.0 mg) and methanol (1 mL) were added to a 25 mL Shrek tube, compound 4 (100.0 mg, 0.31 mmol) was dissolved in methanol (3 mL), the above mixture was added, and the reaction was stirred for 8 h. After the reaction was complete, the reaction mixture was filtered, concentrated under reduced pressure, and finally purified by silica gel column chromatography (dichloromethane / methanol=10:1) to obtain (2R,5R)-Musclide-A1 (57.0mg, yield 81%), It is white powder. [α] D 21 =–8.5 (c 0.5, CHCl 3 ). 1 H NMR (300MHz, CD 3 OD)δ4.19–4.13(m,1H),3.74–3.68(m,1H),2.13–2.01(m,1H),1.77–1.53(m,4H),1.15(d,J=6.2Hz,3H ),0.96(d,J=5.6Hz,3H),0.93(d,J=5.6Hz,3H). 13 C NMR (75MHz, CD 3 OD) δ83.8, 67.1, 34.2, 30.4, 26.1, 21.8, 16.6, 16.3. HRMS (APCI-TOF): calcd for C 8 h 17 o 5 S[M-H] - 225.0791, found 225.0790.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com