Method for synthesizing goserelin impurities
A synthesis method and the technology of goserelin are applied in the preparation methods of peptides, chemical instruments and methods, production of bulk chemicals, etc., which can solve the problems of many reaction steps, complicated operations such as washing and rotary evaporation, and improve the yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
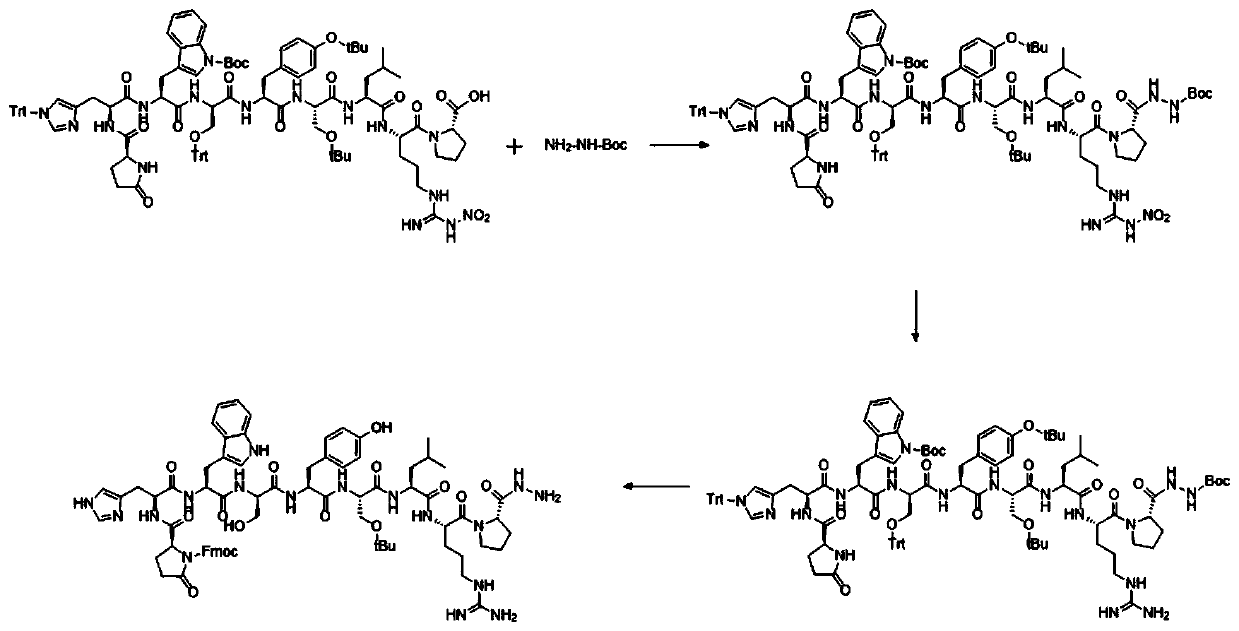
[0038] A kind of synthetic method of goserelin impurity, flow process is as follows figure 1 shown, including the following steps:
[0039] step one:
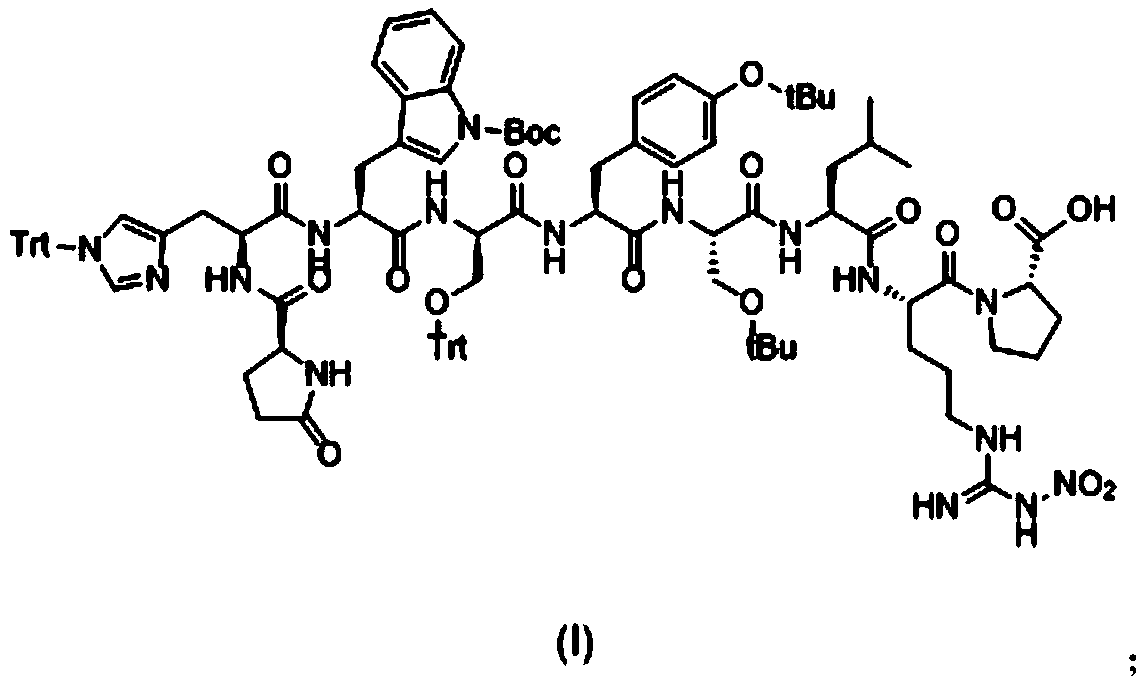
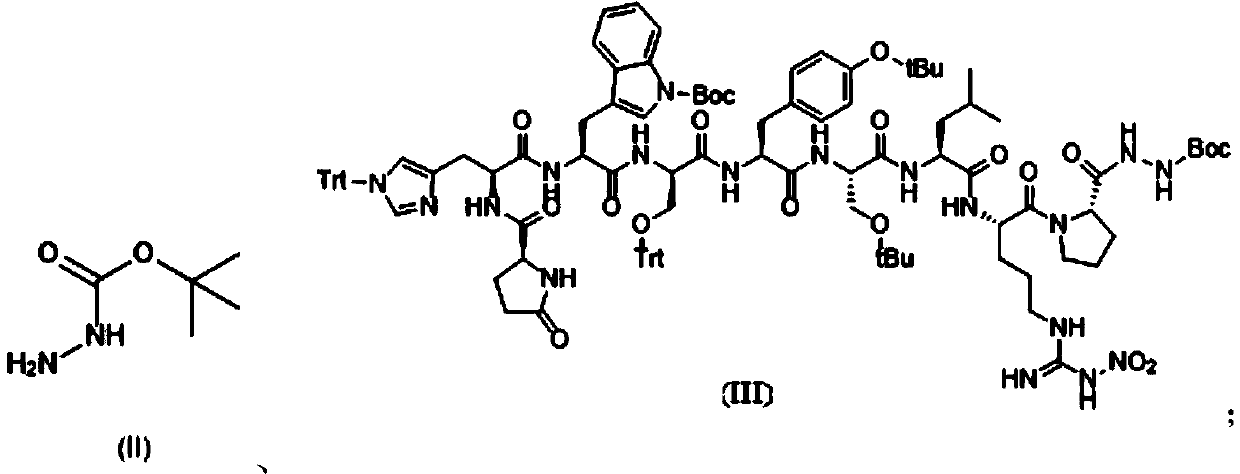
[0040] Synthesis of Pyr-His(Trt)-Trp(Boc)-Ser(Trt)-Tyr(tBu)-D-Ser(tBu)-Leu-Arg(NO2)-Pro-OH:
[0041] 1) Add 10g of CTC-Resin with a substitution degree of 0.47mmol / g into a solid-phase reactor, add 2g of Fmoc-Pro-OH and 10ml of DIEA to react for 2 hours, remove the reaction solution, and wash twice with DMF; After Fmoc protection, wash twice with DMF, once with DCM, and twice with DMF;
[0042] 2) Add Fmoc-Arg(NO2)-OH, Fmoc-Leu-OH, Fmoc-D-Ser(tBu)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Ser(Trt)-OH, Fmoc- Trp(Boc)-OH, Fmoc-His(Trt)-OH, Pyr-OH, the reaction time is 2 hours, the deprotection time is 0.5 hours, the reaction end point is determined by the ninhydrin method; after the reaction, DMF Wash twice, once with DCM, twice with DMF;
[0043] 3) After the washed resin was washed twice with methanol, the resin was removed twice with 20...
Embodiment 2
[0059] On the basis of Example 1, replace Step 4 and Step 5 as follows, and the rest of the operations are the same as Example 1.
[0060] Step 4: a cracking method of goserelin impurities, comprising the following steps:
[0061] Pyr-His-Trp-Ser-Tyr-D-Ser(tBu)-Leu-Arg-Pro-NH-NH 2 Synthesis:
[0062] Prepare 50ml of trifluoroacetic acid: water: triisopropylsilane solution, the volume ratio is 80:10:10. 8.1g Pyr-His(Trt)-Trp(Boc)-Ser(Trt)-Tyr(tBu)-D-Ser(tBu)-Leu-Arg-Pro-NH-NH 2 Added to the solution, stirred for 2 hours. Take out the reaction solution, add it to 200ml, stir well, and then rotary evaporate to obtain 3.6g of oily liquid; add 3.6g of oily liquid to 500ml of ether, precipitate and centrifuge, wash with ether three times, and dry under reduced pressure to finally obtain 2.9g of white solid .
[0063] Step 5: Purification of goserelin impurities:
[0064] 2.9g white solid Pyr-His-Trp-Ser-Tyr-D-Ser(tBu)-Leu-Arg-Pro-NH-NH 2 Dissolve with 10% acetonitrile and wat...
Embodiment 3
[0068] On the basis of Example 1, replace Step 4 and Step 5 as follows, and the rest of the operations are the same as Example 1.
[0069] Step 4: a cracking method of goserelin impurities, comprising the following steps:
[0070] Pyr-His-Trp-Ser-Tyr-D-Ser(tBu)-Leu-Arg-Pro-NH-NH 2 Synthesis:
[0071] Prepare 50ml of trifluoroacetic acid: water: triisopropylsilane solution, the volume ratio is 80:10:10. 7.8g Pyr-His(Trt)-Trp(Boc)-Ser(Trt)-Tyr(tBu)-D-Ser(tBu)-Leu-Arg-Pro-NH-NH 2 Added to the solution, stirred for 2 hours. Take out the reaction solution, add it to 200ml, stir well, and rotary evaporate to obtain 3.4g oily liquid; add 3.4g oily liquid to 500ml ether, precipitate and centrifuge, wash with ether three times, and dry under reduced pressure to finally obtain 2.7g white solid .
[0072] Step 5: Purification of goserelin impurities:
[0073] 2.7g white solid Pyr-His-Trp-Ser-Tyr-D-Ser(tBu)-Leu-Arg-Pro-NH-NH 2 Dissolve with 10% acetonitrile and water, filter;
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com