Polypeptide with morphine tolerance and side effect relieving function and application of polypeptide
A technology for morphine tolerance and side effects, applied in the field of medicine, can solve problems such as limiting the clinical application of morphine, and achieve the effect of relieving morphine tolerance and side effects symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
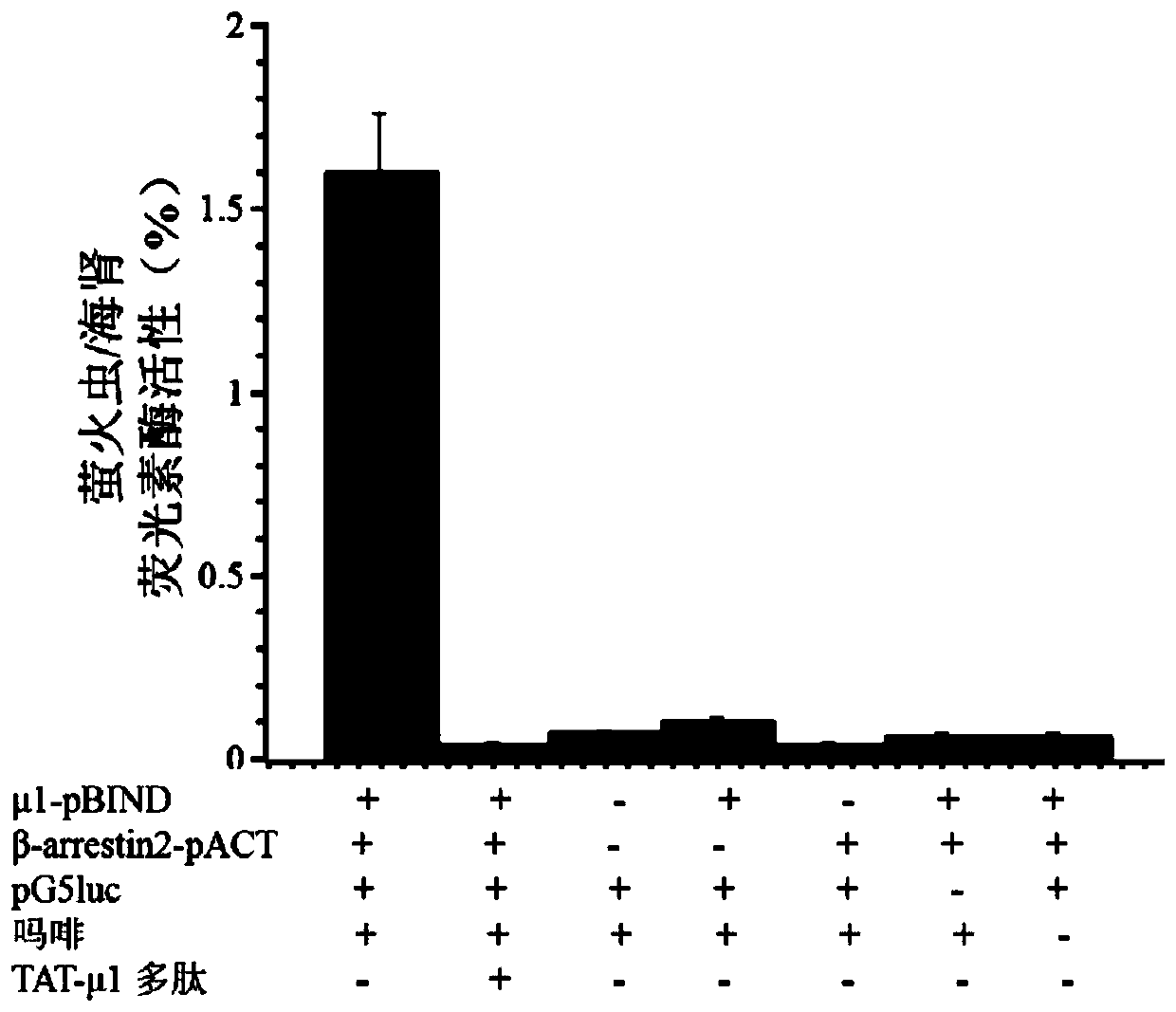
[0033] Example 1 Obtaining of Polypeptide TAT-μ1
[0034] (1) Obtaining TAT-μ1 polypeptide with mold-piercing activity
[0035] Extract the total RNA of SH-SY5Y cells, reverse transcribe to obtain cDNA, and use the cDNA as a template to obtain the mu opioid receptor carboxy-terminal polypeptide sequence by PCR, its upstream primer: 5'CCATCTCGAGATGTACGGTCGTAAAAAACGTCGTCAGCGTCGTCGTATCCCAACCTCTTCCAACATTG3'(SEQ ID NO.7), downstream primer 5' CGTCGGATCCGGGCAACGGAGCAGTTTCTG 3'(SEQ ID NO.8) (bold is the penetrating peptide sequence), insert the TAT coding sequence into the upstream primer, add XhoI and BamHI restriction sites to the upstream and downstream primers respectively, and link by restriction enzymes, The pWaldo-TAT-μ1 E. coli expression vector was constructed and transformed into BL21(DE3) expression strain to obtain the recombinant.
[0036] Inoculate the BL21(DE3) recombinant strain in 200ml LB medium and culture overnight at 37°C. Transfer 50ml overnight culture to 1L ...
Embodiment 2
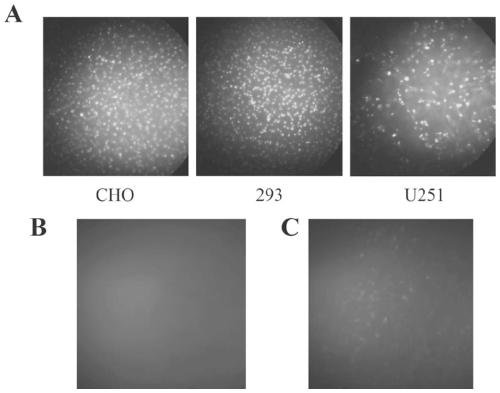
[0043] Example 2 Application Research of Polypeptide TAT-μ1
[0044] Frozen section preparation: ①The mice in each experimental group were anesthetized with 10% chloral hydrate (350mg / kg, i.p.), opened their thoracotomy, and quickly perfused the left ventricle with 150ml normal saline (adjusted to 37°C in advance), followed by perfusion with 4% paraformaldehyde phosphate 500ml of salt buffer solution (pre-cooled to 0°C-4°C), the perfusion should be completed within 1 hour. After the perfusion, the brain was removed and fixed in 4% paraformaldehyde solution at 4°C for 8 hours. ②Put the fixed brains in 20% and 30% sucrose phosphate buffer in sequence, dehydrate in gradient, and soak until the tissue sinks to the bottom of the bottle, so as to avoid the formation of ice crystals during rapid freezing and improve the quality of sectioning. ③Slice with a cryostat, each slice is 25 μm. ④ Wash the slices with 10mM PBS 3 times, 5min each time, seal the slides, and take pictures to d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com