Application of tamoxifen, rapamycin and ginsenoside Rg3 composition in preparing medicaments for treating liver cancer
A technology of tamoxifen and ginsenoside, which is applied in drug combinations, antineoplastic drugs, and pharmaceutical formulations, can solve the problems of high recurrence rate, little curative effect enhancement, slow postoperative healing, etc., to reduce toxic and side effects, Significant curative effect, the effect of inhibiting the formation of tumor blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1. Reagents: RPMI-1640 medium is a product of GIBCOL BRL Company in the United States; mouse anti-survivin and p70s6k Thr389 monoclonal antibodies were purchased from Cell Signal Company; secondary antibody HRP-labeled anti-mouse IgG and chemiluminescence (ECL) substrate were purchased from Santa Cruz Company; tamoxifen, rapamycin, and ginsenoside Rg3 were all purchased from Sigma Company, and Renilla luciferase plasmid pRL-CMV was purchased from Promega Company. Plasmid pLUC encoding firefly luciferase gene and survivin promoter sequence was donated by Dr. Xun from University of North Carolina, USA.
[0017] 2. Cell culture: The human liver cancer HepG2 cell line was routinely cultured in RPMI-1640 culture medium, adding 10% calf serum, human penicillin (concentration 100U / ml) and streptomycin (concentration 100 μg / ml), 37°C, 5%CO 2 cultured in an incubator. After 3-4 generations of adherent growth in the incubator, the cells in the logarithmic growth phase were take...
Embodiment 2
[0029] Tamoxifen has achieved obvious curative effect in inhibiting tumor growth in breast cancer and liver cancer experiments, and tamoxifen combined with rapamycin and ginsenoside Rg3 can enhance the effect of its cytotoxic drugs. Therefore, the combined therapy of tamoxifen combined with rapamycin and ginsenoside Rg3 will provide an efficient, low-toxic and cheap means for the treatment of liver cancer.
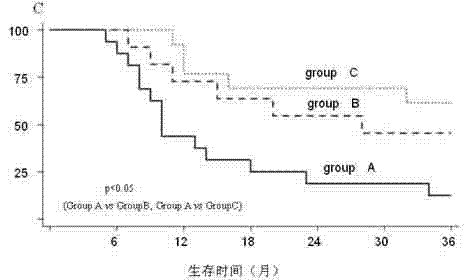
[0030] We have carried out clinical detection of survivin, p-Akt, and ER expression in tissue samples after liver cancer surgery in more than 100 cases. For patients with positive expression of survivin and p-Akt, moxifen combined with rapamycin and ginsenoside were partly used during outpatient follow-up. Rg3, tamoxifen monotherapy and control observation and follow-up, at least 2 courses of treatment. The results showed that: compared with the monotherapy group and the observation group (the control group was not administered), the three-drug combination therapy group (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com