Copper corrosion inhibitor based on metal organic frame material MOFs and preparation method thereof
A metal-organic framework and copper corrosion inhibitor technology, which is applied in the field of copper corrosion inhibitors, can solve the problems of few types of copper corrosion inhibitors and low corrosion inhibition performance, and achieve significant corrosion inhibition effects, small dosage, and corrosion inhibition effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
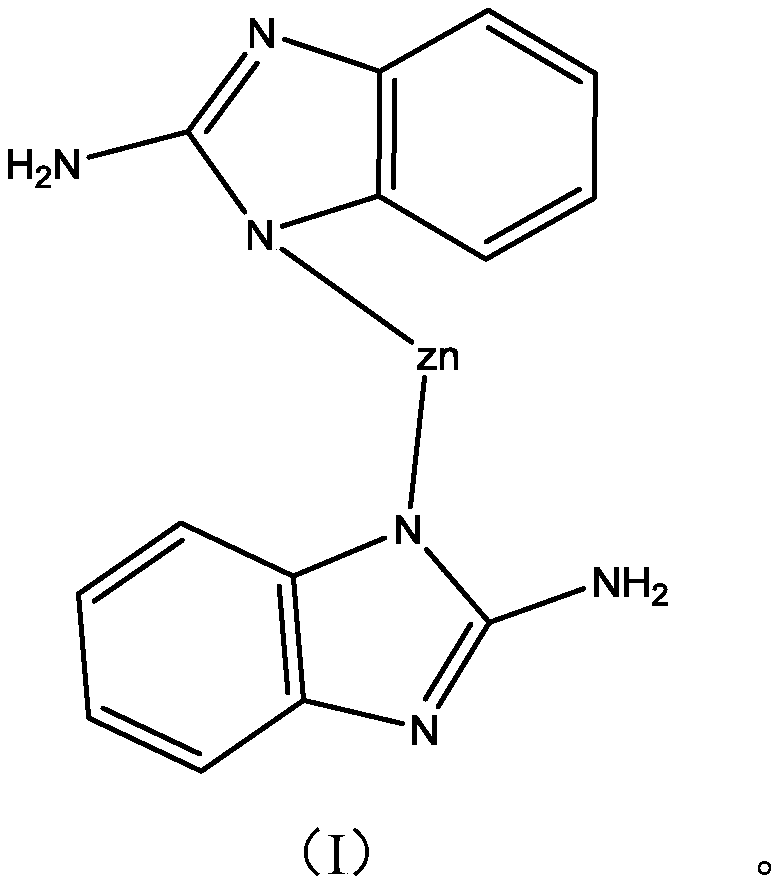
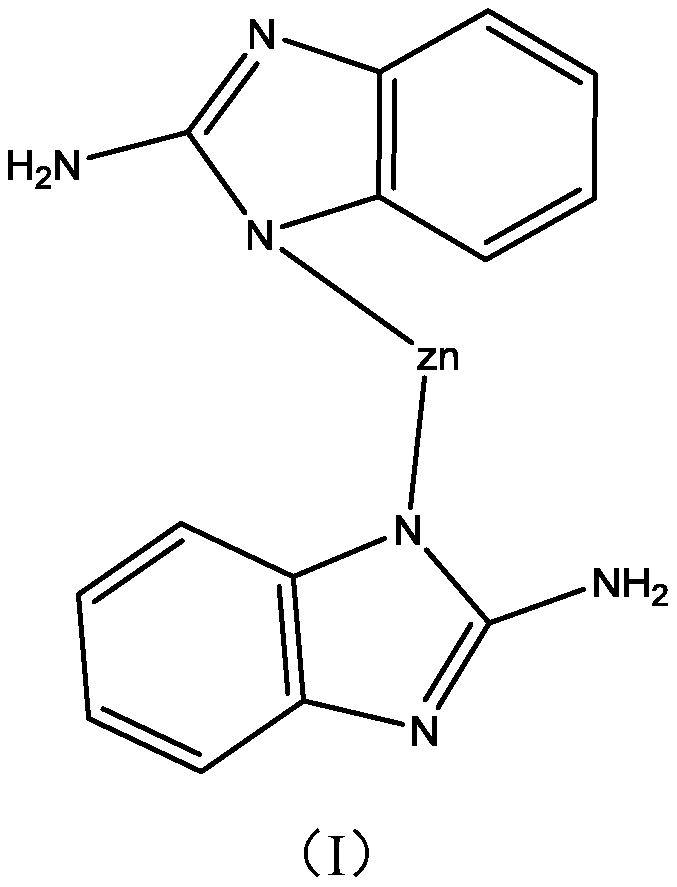
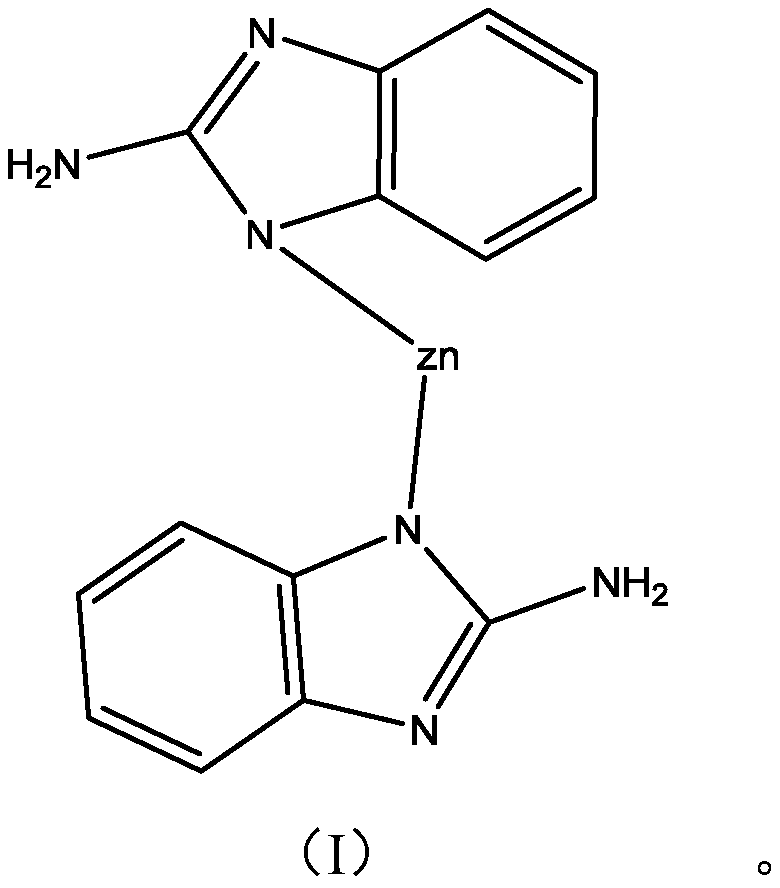
[0029] Put 56 grams of zinc nitrate, 50 grams of 2-aminobenzimidazole and 800 mL of N, N-dimethylformamide into a beaker and stir to dissolve them. After mixing evenly, transfer them to a closed hydrothermal kettle for a heated solvothermal reaction , when the reaction temperature reaches 80°C, maintain the temperature for 1 hour; continue to raise the temperature to 140°C, maintain the temperature for 24 hours to obtain aminobenzimidazole / Zn 2+ MOFs. Add 20 grams of aminobenzimidazole / Zn prepared above in the beaker 2+ MOFs, 40 grams of isopropanol, and 40 grams of N,N-dimethylformamide to obtain 100 grams of copper corrosion inhibitor.
[0030] Corrosion inhibition performance evaluation:
[0031] At 25°C, in 0.5M NaCl solution, the corrosion inhibition performance of the copper corrosion inhibitor of the present invention on red copper was evaluated by the weight loss test method, and the weight loss test time was 72 hours. Table 1 shows the comparative evaluation result...
Embodiment 2
[0036] Put 28 grams of zinc sulfate, 60 grams of 2-aminobenzimidazole and 700 mL of N, N-dimethylformamide into a beaker and stir to dissolve them. After mixing evenly, transfer them to a closed hydrothermal kettle for a heated solvothermal reaction. , when the reaction temperature reaches 60°C, maintain the temperature for 0.5 hours; continue to raise the temperature to 120°C, maintain the temperature for 18 hours to obtain aminobenzimidazole / Zn 2+ MOFs.
[0037] Add 40 grams of aminobenzimidazole / Zn prepared above in the beaker 2+ MOFs, 30 grams of methanol, 30 grams of formamide to obtain 100 grams of copper corrosion inhibitor.
[0038] Corrosion inhibition performance evaluation:
[0039] At 50°C, in 0.3M NaCl solution, the corrosion inhibition performance of the copper corrosion inhibitor of the present invention on red copper was evaluated by the weight loss test method, and the weight loss test time was 72 hours. Table 2 shows the comparative evaluation results of t...
Embodiment 3
[0044] Put 60 grams of zinc nitrate, 65 grams of 2-aminobenzimidazole and 1000 mL of N, N-dimethylformamide into a beaker and stir to dissolve them. After mixing evenly, transfer them to a closed hydrothermal kettle for a heated solvothermal reaction. , when the reaction temperature reaches 90°C, maintain the temperature for 2 hours; continue to raise the temperature to 160°C, maintain the temperature for 36 hours to obtain aminobenzimidazole / Zn 2+ MOFs.
[0045] Add 30 grams of aminobenzimidazole / Zn prepared above in the beaker 2+ MOFs, 40 grams of ethanol, 30 grams of formamide to obtain 100 grams of copper corrosion inhibitor.
[0046] Corrosion inhibition performance evaluation:
[0047] At 60°C, in 0.2M NaCl solution, the corrosion inhibition performance of the copper corrosion inhibitor of the present invention on red copper was evaluated by the weight loss test method, and the weight loss test time was 72 hours. Table 3 shows the comparative evaluation results of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com