A kind of anti-tuberculosis mycobacterium cfp-10 protein monoclonal antibody and application thereof
A CFP-10, monoclonal antibody technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 immunogen preparation
[0026] Using the DNA of Mycobacterium tuberculosis H37Rv strain as a template, the amplification primers were designed for the open reading frame region of CFP-10 as shown in Table 1. PCR amplification was performed, and the fragment was digested and connected into the prokaryotic expression vector pBVIL1 to construct the pBVIL1-CFP-10 recombinant plasmid.
[0027] Table 1 Amplification primers of CFP-10 fragment
[0028]
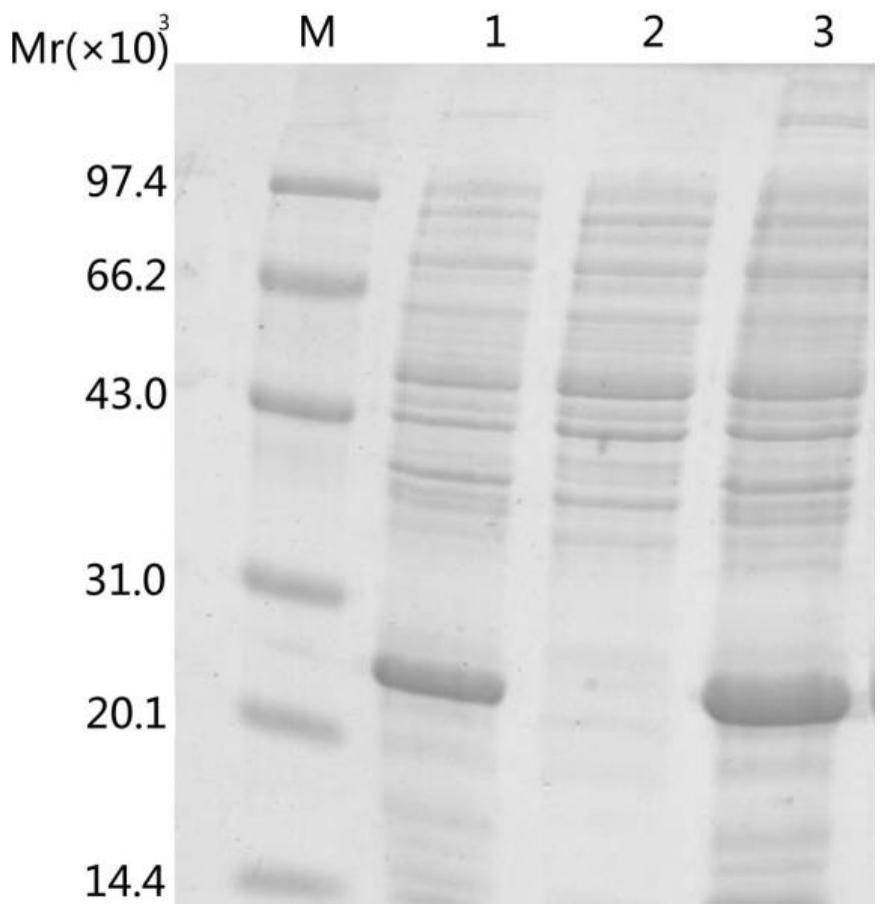
[0029] Transform the recombinant expression plasmid with correct sequencing into HB101 competent cells, pick a single colony in 3ml LB liquid medium containing ampicillin sodium, shake and culture overnight at 37°C, inoculate in fresh LB liquid medium the next day and cultivate until After the logarithmic phase, induce overnight at a temperature of 42°C. Perform SDS-PAGE gel electrophoresis to analyze the expression of the inclusion body of the target protein in the precipitate, and the results are as follows...
Embodiment 2
[0031] Embodiment 2 hybridoma cell lines are established
[0032] Eight-week-old BALB / C male mice were used to inject CFP-10 antigen plus an equal amount of Freund's complete adjuvant into the back and intraperitoneal injection of mice (50 μg / mouse); the second and third immunizations with the same dose were carried out at the fourth and eighth weeks. With Freund's incomplete adjuvant, spleen cells were taken for fusion after 3 days.
[0033] Resuscitate SP20 myeloma cells so that they are in logarithmic growth phase. The immunized BALB / c mice were taken, and the eyeballs were removed to collect blood for the positive control serum. At the same time, the mice were killed by cervical dislocation, and the body surface was disinfected with 75% alcohol for 3-5 minutes. The spleen was collected to prepare a spleen cell suspension.
[0034] Take the above splenocytes and myeloma cells according to the ratio of 5:1, mix them in serum-free DMEM medium, centrifuge at 1500rpm for 5 min...
Embodiment 3
[0036] Example 3 Preparation of monoclonal antibody and its subtype analysis
[0037] The C-10 hybridoma cells were revived and cultured in 1640 medium containing 10% fetal bovine serum. Each BALB / c mouse was intraperitoneally injected with 0.5ml of liquid paraffin. Collect the cells after 10 days, resuspend the cells with 10ml normal saline, and inject 0.5ml intraperitoneally into each mouse (the cell density is about 1×10 7 pieces / ml). After 2 weeks, ascitic fluid was collected.
[0038] 1) Purification of monoclonal antibodies
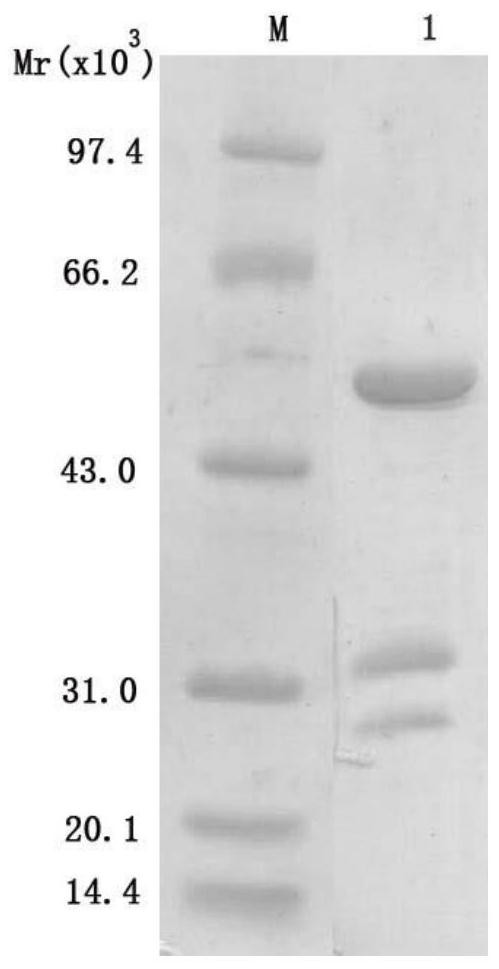
[0039] Antibody purification was carried out with Melon Gel Monoclonal IgG Purification Kit from Thermo Company, and the results were as follows figure 2 shown. The purified antibody was stored at -20°C after aliquoting.
[0040] 2) Subclass identification
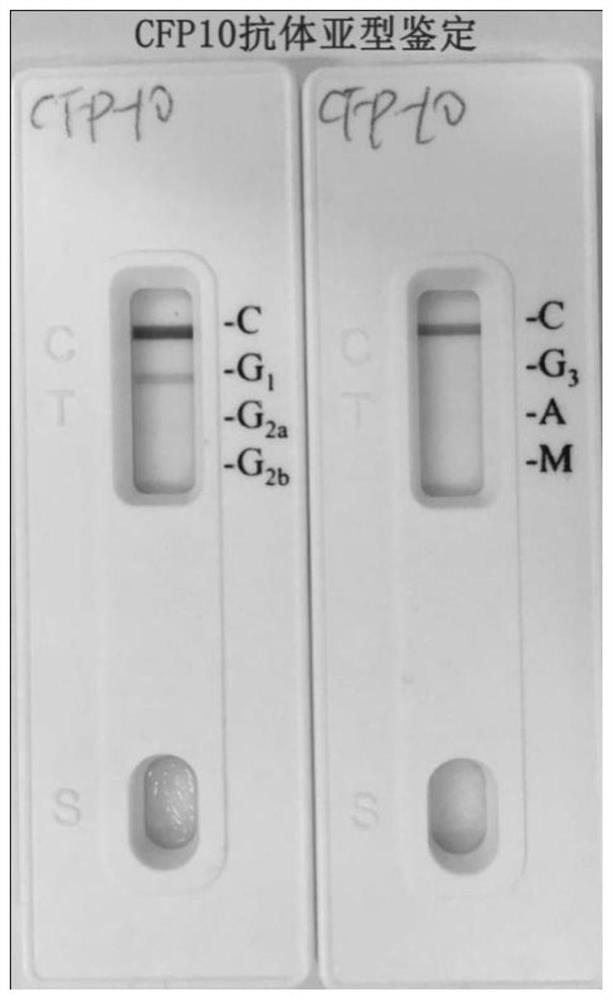
[0041] Using the Pierce Papid Isotyping Kit-Mouse kit antibody subtype identification, the monoclonal antibody was identified as the mouse IgG1 subtype, such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com