Phenyl boronic acid-based polymer carrier and applications in selective adsorption of saccharide-based substances
A phenylboronic acid-based, polymer technology, applied in the direction of solid adsorbent liquid separation, application, sugar production, etc., can solve the problems of potential safety hazards, low recovery rate, large consumption, etc., and achieve selective adsorption and rapid adsorption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
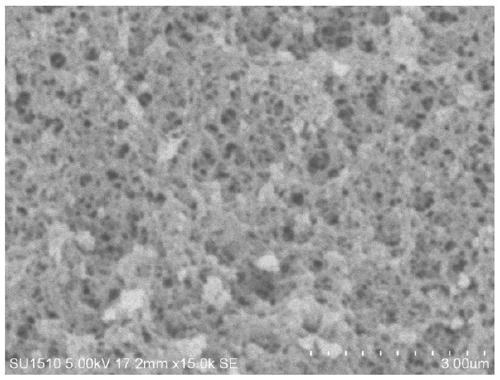
[0060] Embodiment 1: Preparation of phenylboronic acid-based polymer carrier
[0061] Dissolve 1 mol of phenylboronic acid-based monomer AAPBA and 10 mol of cross-linking agent EGDMA into 1000 mL of DMSO, shake and mix evenly, and after standing in the dark for 1 hour, add 5 g of initiator azobisisobutyronitrile previously dissolved in 10 mL of DMSO, After oscillating evenly, ultrasonic degassing was performed for 30 minutes, and then nitrogen gas was continuously introduced for 30 minutes. After vacuum sealing, it was placed in a water bath at 60°C for 12 hours.
[0062] The block polymer obtained is ground, pulverized, sieved to obtain 120 mesh and 100 mesh size particles, the above particles are wrapped with filter paper, placed in a Soxhlet extractor, add 20% acetic acid: 80% methanol (v / v) extracting solution at 80°C for 24h to remove the porogen and solvent, then use 100% methanol to continue the extraction for 2h, and dry the obtained polymer particles at 60°C for 24h ...
Embodiment 2
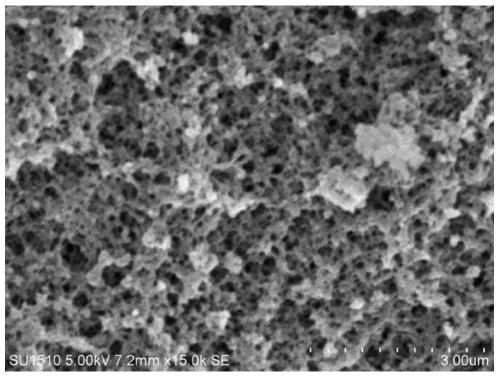
[0064] Embodiment 2: Preparation of phenylboronic acid-based polymer carrier
[0065] Shake and mix 1mol of AAPBA with 2000mL of DMSO evenly, and shake in the dark for 12 hours to make the AAPBA evenly dispersed, then add 5mol of EGDMA, shake evenly and keep it in the dark for 2 hours, then add the initiator azodiisocyanate dissolved in 20mL of DMSO in advance Butyronitrile 10g, after shaking evenly, ultrasonic degassing for 10min, then continuously blowing in nitrogen for 15min, after vacuum sealing, place in 70°C water bath for 8h.
[0066] The block polymer obtained is ground, pulverized, sieved to obtain 120 mesh and 100 mesh size particles, the above particles are wrapped with filter paper, placed in a Soxhlet extractor, add 20% acetic acid: 80% methanol (v / v) of the extract was extracted at 90°C for 48h, the porogen DMSO was removed, and then 100% methanol was used to continue the extraction for 10h, and then the obtained polymer particles were dried under reduced press...
Embodiment 3
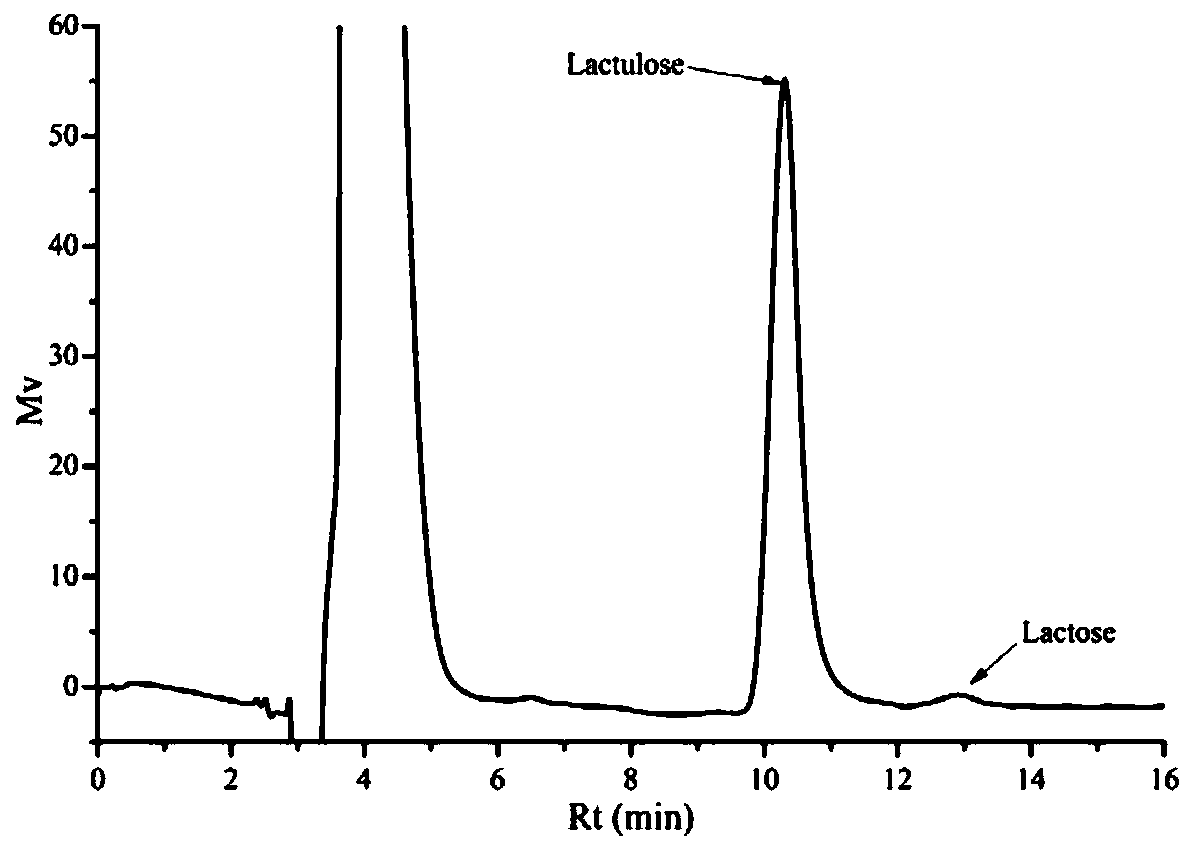
[0068] Embodiment 3: Batch type separation and purification of lactulose
[0069] Formulate a 5:25 mg / mL solution of lactulose and lactose at a ratio of 1:5, and then add 25 g of the 100-mesh phenylboronic acid-based polymer carrier prepared in Example 1 to the above 1L lactulose-lactose solution , at pH 7.0, oscillating and adsorbing at 25°C for 30min, and filtering and separating to obtain the adsorbed phenylboronic acid-based polymer carrier, and then adding the adsorbed carrier to 100mL deionized deionized water (pH ~ 5.8), at 25°C, Desorb for 15 minutes under the condition of 150r / min, and take samples to measure the adsorption capacity and the purity of the desorbed solution. The final detection of the phenylboronic acid-based polymer carrier adsorbed lactulose load is 79mg / g dry basis carrier, while the adsorption capacity of lactose is only 1.5mg / g dry basis carrier, the desorption rate is 90%, and the HPLC collection of desorption liquid is as follows: image 3 As sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com