Organic porous polymer as well as preparation and application thereof
A porous polymer, organic technology, applied in the field of porous structure polymer organic electroluminescent materials, can solve problems such as low efficiency, waste of time, increase experimental cost, etc., to improve efficiency, simplify the production process, and promote energy transfer. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
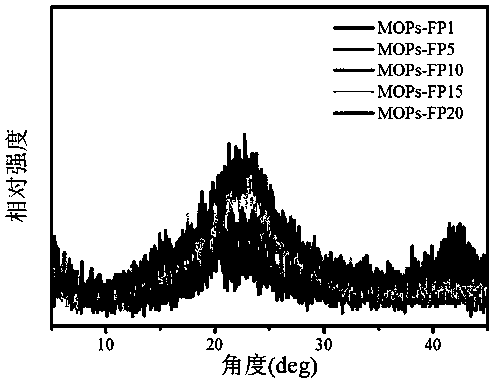
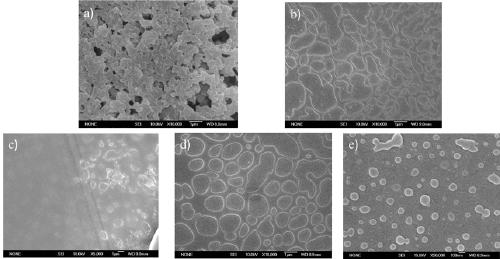
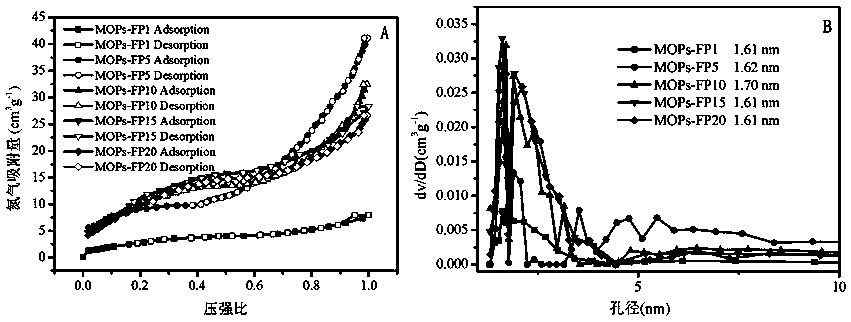
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of an organic porous polymer in which the construction monomer 1,3,6,8-tetrasubstituted pyrene accounts for 1% of the total molar weight of the polymer.
[0052] 1), the synthesis of 1,3,6,8-tetrabromopyrene.
[0053]
[0054] Add 3.26g (10mmol) pyrene to a 250ml three-necked flask, vacuumize and ventilate 2 Each time 3 times, the air in the flask was fully exhausted. Add 50ml of THF, stir and mix at room temperature for 30min, add 0.6g (25mmol) NaH into the flask in 5 times, with an interval of 15min between each dosing. Vacuum again and pass N 2 , Dissolve 1.70g (4mmol) pentaerythritol bromide in 20ml THF, slowly drop it into a three-necked flask through a normal pressure separatory funnel within 30min, heat to 50°C for 6h, then raise the temperature to 75°C for 24h.
[0055] After the reaction, the mixed solution was rotary evaporated to remove THF, water was added, and CH 2 Cl 2 Extract 3 times, collect CH 2 Cl 2 phase, dried over anhy...
Embodiment 2
[0063] Example 2: Synthesis of an organic porous polymer in which the building monomer 1,3,6,8-tetrasubstituted pyrene accounts for 5% of the total molar weight of the polymer.
[0064] Weigh 0.23g (0.42mmol) of M1, 0.34g (0.53mmol) of M2 and 0.0259g (0.05mmol) of M3, mix them into a 250ml three-necked flask, vacuum and ventilate 2 Each 3 times to exhaust the air in the flask. Add 30ml of toluene (water removed in advance) to the three-necked flask, and stir for 10min. Weigh 5.0g K 2 CO 3 Dissolve in 15ml of water, measure 1ml of Aliquant 336 phase transfer catalyst and dissolve in 5ml of anhydrous toluene, respectively in N 2 Put it into a three-necked flask under protection, vacuumize and pass N 2 1 time each. Weigh 0.05 g of tetrakis(triphenylphosphine) palladium catalyst into a three-necked flask, heat to 100° C., and stop the reaction after 3 days of reflux reaction.
[0065] The reaction liquid was cooled to room temperature, added water, extracted with toluene, th...
Embodiment 3
[0067] Example 3: Synthesis of an organic porous polymer in which the building monomer 1,3,6,8-tetrasubstituted pyrene accounts for 10% of the total molar weight of the polymer.
[0068] Weigh M1 0.19g (0.35mmol), M2 0.35g (0.55mmol), M3 0.0518g (0.1mmol), mix them into a 250ml three-necked flask, vacuum and vent 2 Each 3 times to exhaust the air in the flask. Add 30ml of toluene (water removed in advance) to the three-necked flask, and stir for 10min. Weigh 5.0g K 2 CO 3 Dissolve in 15ml of water, measure 1ml of Aliquant 336 phase transfer catalyst and dissolve in 5ml of anhydrous toluene, respectively in N 2 Put it into a three-necked flask under protection, vacuumize and pass N 2 1 time each. Weigh 0.05 g of tetrakis(triphenylphosphine) palladium catalyst into a three-necked flask, heat to 100° C., and stop the reaction after 3 days of reflux reaction.
[0069] The reaction liquid was cooled to room temperature, added water, extracted with toluene, the extract was rot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com