Haemostatic sterilizing dressing and preparation method thereof
A technology of formula and mixture, which is applied in the field of medical materials, can solve the problems of affecting the effect of patient rescue, single bactericidal effect, wound suppuration, etc., and achieve the effect of preventing festering or infection, good bactericidal effect, and good hemostatic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
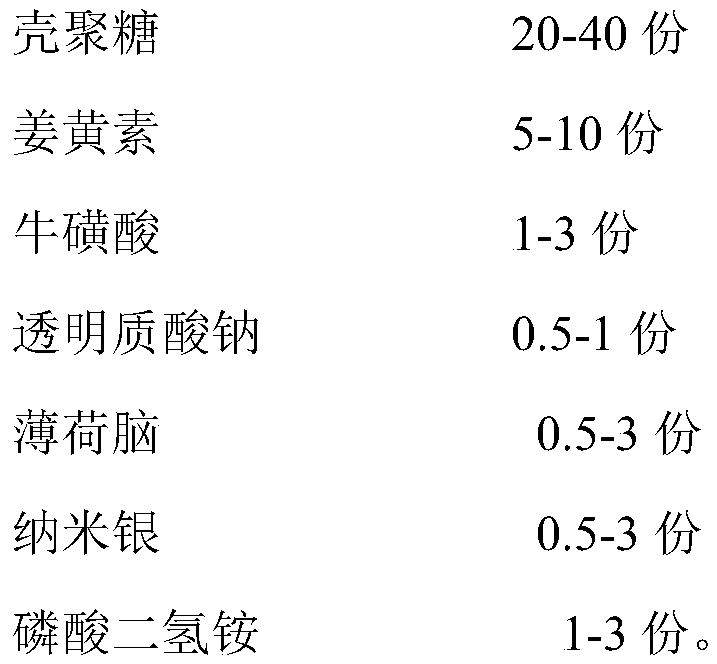
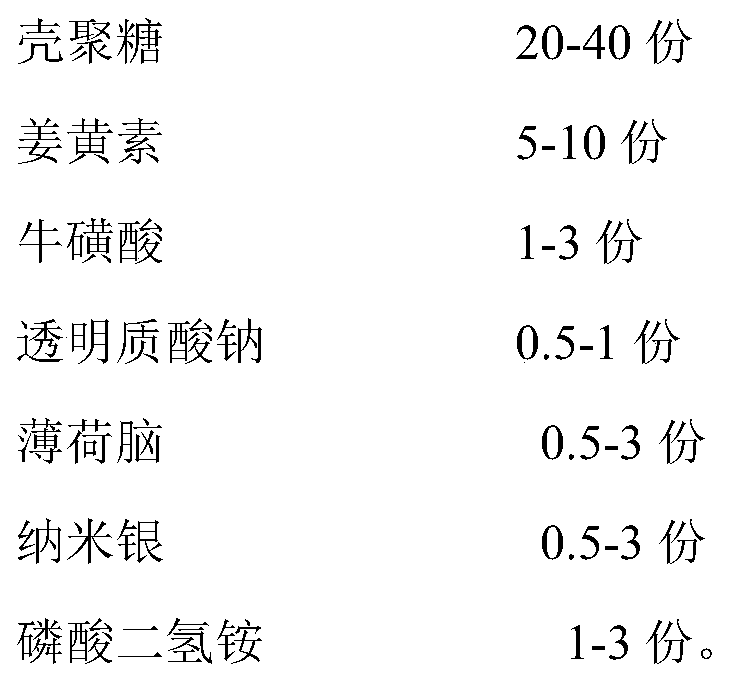
[0037] A hemostatic and antiseptic dressing, comprising the following components in parts by weight:
[0038]
[0039] The size of the nano-silver is 20-40nm.
[0040] A kind of its preparation method of hemostatic, antiseptic dressing, comprises the following steps:
[0041] (1) Take each component according to the formula quantity, dissolve chitosan, curcumin, sodium hyaluronate, and menthol in 50 parts of water, mix and stir to prepare mixture A, and set aside;
[0042] (2) Taurine, nano-silver, and ammonium dihydrogen phosphate are added to 10 parts of water and stirred to prepare mixture B for subsequent use;
[0043] (3) Mix mixture A and mixture B and stir to prepare mixture C, and then soak the medical gauze into mixture C
[0044] Soak it at 25°C for 24 hours, take out the gauze, and dry it to obtain the hemostatic and sterilizing dressing.
[0045] The stirring speed in the step (1) is 400 rpm, and the stirring time is 30 minutes.
[0046] The temperature of t...
Embodiment 2
[0051] A hemostatic and antiseptic dressing, comprising the following components in parts by weight:
[0052]
[0053] The dressing also includes 1 part of zinc oxide in parts by weight.
[0054] The size of the nano silver is 40-60nm.
[0055] A kind of its preparation method of hemostatic, antiseptic dressing, comprises the following steps:
[0056] (1) Take each component according to the formula quantity, dissolve chitosan, curcumin, sodium hyaluronate, and menthol in 80 parts of water, mix and stir to prepare mixture A, and set aside;
[0057] (2) Taurine, nano silver, ammonium dihydrogen phosphate, zinc oxide are added to 15 parts of water and stirred to prepare mixture B,
[0058] spare;
[0059] (3) Mix mixture A and mixture B and stir to prepare mixture C, and then soak the medical gauze into mixture C
[0060] Soak it at 30°C for 18 hours, take out the gauze, and dry it to obtain the hemostatic and sterilizing dressing.
[0061] The stirring speed in step (1)...
Embodiment 3
[0067] A hemostatic and antiseptic dressing, comprising the following components in parts by weight:
[0068]
[0069]
[0070] A kind of its preparation method of hemostatic, antiseptic dressing, comprises the following steps:
[0071] (1) Take each component according to the formula quantity, dissolve chitosan, curcumin, sodium hyaluronate, and menthol in 100 parts of water, mix and stir to prepare mixture A, and set aside;
[0072] (2) Taurine, nano-silver, and ammonium dihydrogen phosphate are added to 20 parts of water and stirred to prepare mixture B for subsequent use;
[0073] (3) Mix mixture A and mixture B and stir to prepare mixture C, and then soak the medical gauze into mixture C
[0074] Soak it at 40°C for 12 hours, take out the gauze, and dry it to obtain the hemostatic and sterilizing dressing.
[0075] The stirring speed in the step (1) is 200 rpm, and the stirring time is 50 minutes.
[0076] The temperature of the water described in step (2) is 70°C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com