Preparation method of anti-AMH specific antibody and application of antibody in AMH detection kit
A specific and antibody technology, applied in the application of antibodies in AMH detection kits and the preparation of anti-AMH specific antibodies, can solve the problems of not providing further antibody evaluation results, underestimation of AMH detection concentration, and low sensitivity of detection methods. , to achieve the effect of reliable clinical diagnosis and treatment, simple preparation method and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
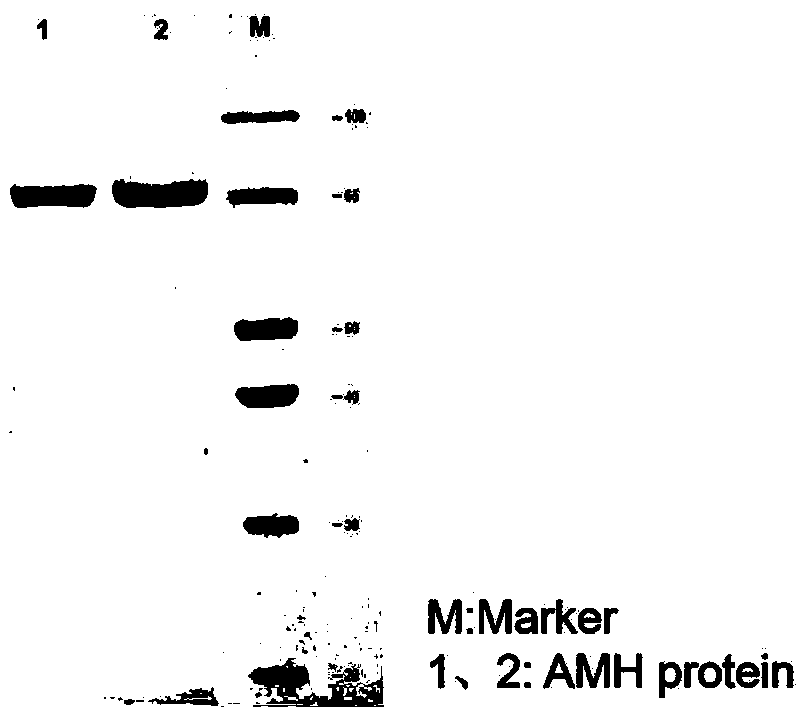
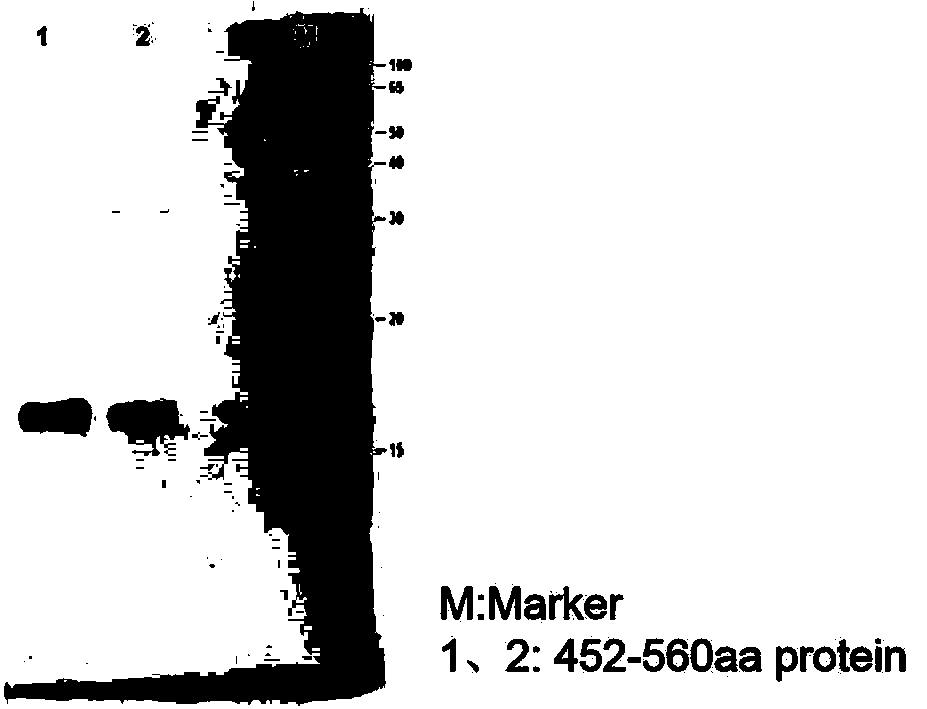
[0024] Example 1 Preparation of AMH full-length protein and mature region fragments
[0025] 1. Whole gene synthesis of AMH coding sequence
[0026] The mRNA sequence (NM_000479.4) of AMH was queried from NCBI (National Center for Biotechnology Information), and its expressed sequence was (SEQ ID NO.1).
[0027] Entrusted Shanghai Sangon Bioengineering Co., Ltd. to carry out the whole gene synthesis.
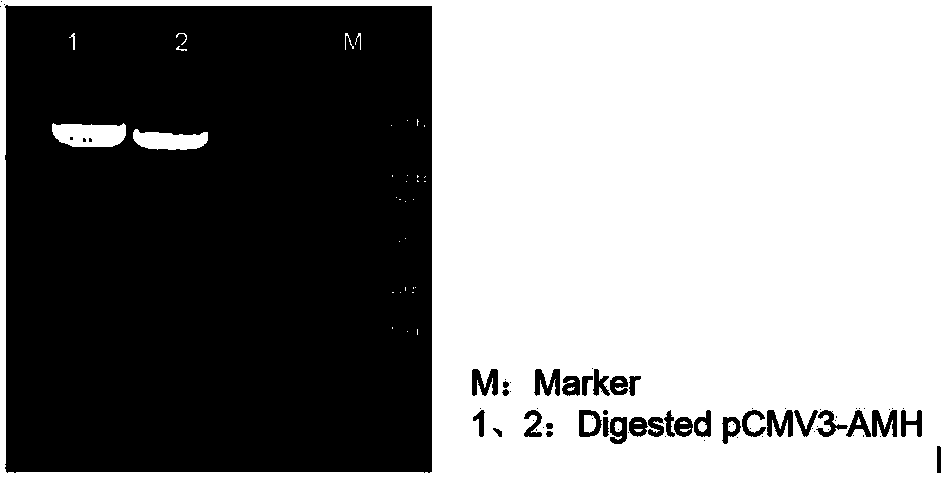
[0028] 2. Construction and identification of recombinant human AMH expression vector
[0029] Using the synthetic AMH gene as a template, the amplification primers containing KpnI and XbaI restriction enzyme cleavage sites were designed respectively, and a His tag sequence was introduced into the upstream primers for later purification. The target gene was amplified using amplification primers, and a fusion fragment with the structure of "KpnI restriction site-His tag-AMH sequence-XbaI restriction site" was amplified. Subsequently, KpnI and XbaI were used to perform double en...
Embodiment 2
[0035] Example 2 Preparation of mouse monoclonal antibody against AMH
[0036] 1. Immunization of mice
[0037] After fully emulsifying the AMH recombinant protein obtained in Example 1 with Freund's complete adjuvant, intraperitoneal immunization of 5-week-old female Balb / c mice, the initial dose is 100ug / mouse; the interval after the first immunization is 21 days The second and third immunizations were carried out in 42 days respectively, and the immunization dose was 50ug / mouse. About 10 days after the third immunization, the blood was collected from the tail, and the serum titer was detected by the indirect method using a 96-well plate coated with AMH.
[0038] 2. Preparation of hybridoma cells
[0039] Select the indirect method to detect serum titer greater than 10 4 Intrasplenic booster immunization was carried out in the mice, and the immunization dose was 100ug / mouse. Three days after the booster immunization, the mouse spleen was fused with mouse myeloma cell NS1...
Embodiment 3
[0049] Example 3 Establishment of the ELISA method for the detection of AMH by the double-antibody sandwich method
[0050] 1. Magnetic particle coating
[0051] Take 30 μl of the mixed magnetic particle stock solution and wash it 5 times with 300 μl of PBS buffer solution, then activate the magnetic particles with 10% glutaraldehyde for 1 hour, and then use pH 7-8 PBS buffer solution to activate the magnetic particles. Microparticles were washed 2 times. Anti-AMH mouse monoclonal antibody AMH-2# was added to the magnetic beads at an amount of 0.3 μg / person, coated at 4°C for 2 hours, and finally blocked with BSA-containing blocking solution for 2 hours.
[0052] 2. HRP-labeled anti-AMH mouse monoclonal antibody AMH-1#
[0053] The anti-AMH mouse monoclonal antibody AMH-1# was labeled with HRP (Roche) at a mass ratio of 1.5:1. The HRP-labeled anti-AMH mouse monoclonal antibody AMH-1# was diluted to 1:4000 with diluent.
[0054] 3. Clinical sample testing
[0055] Collect ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com