Flame retardant containing multivalent phosphorus element and preparation method thereof
A technology of phosphorus element and multivalent state, which is applied in the field of phosphorus-containing flame retardants, can solve the problems that need to be further improved, and achieve the effect of high flame retardant efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
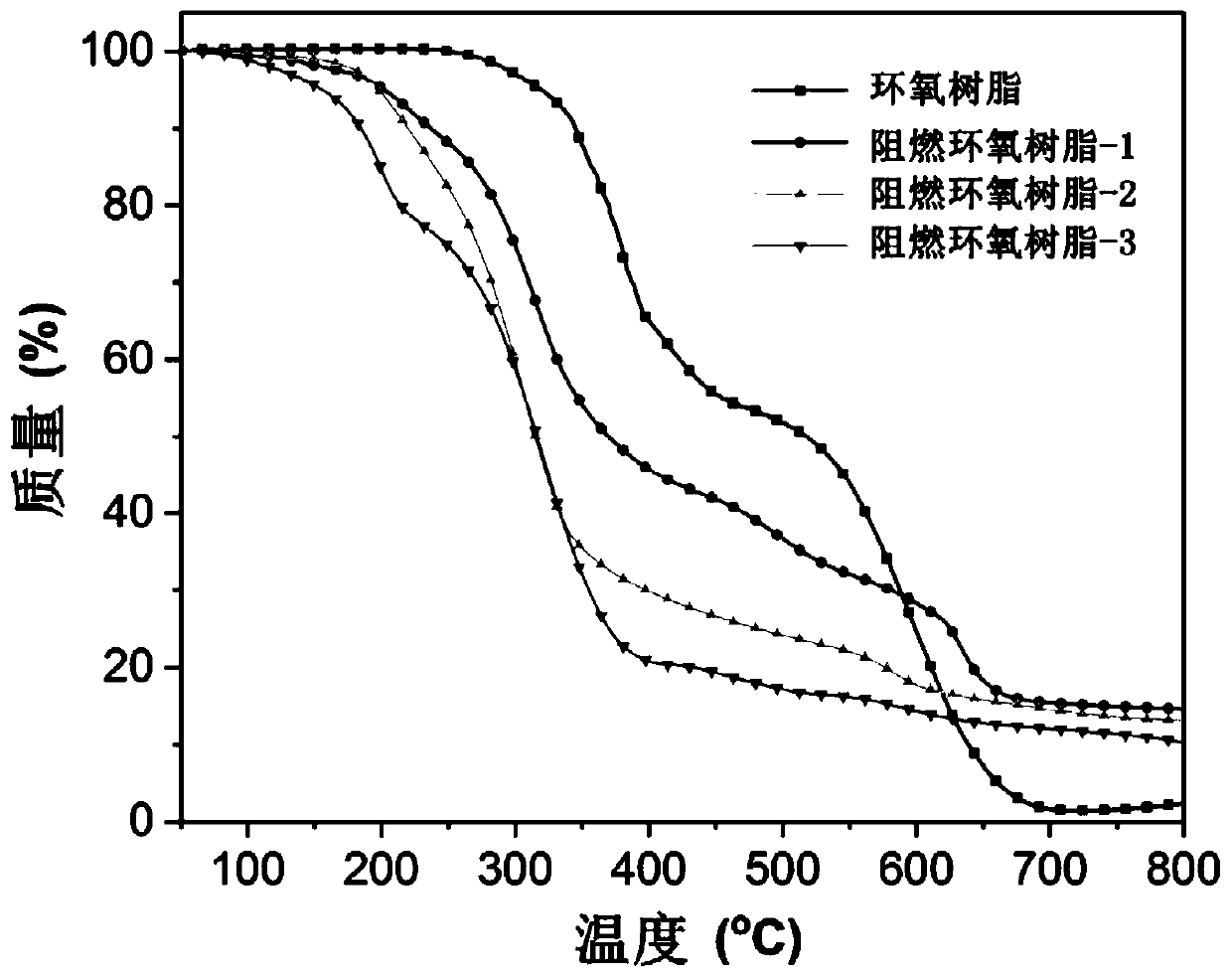
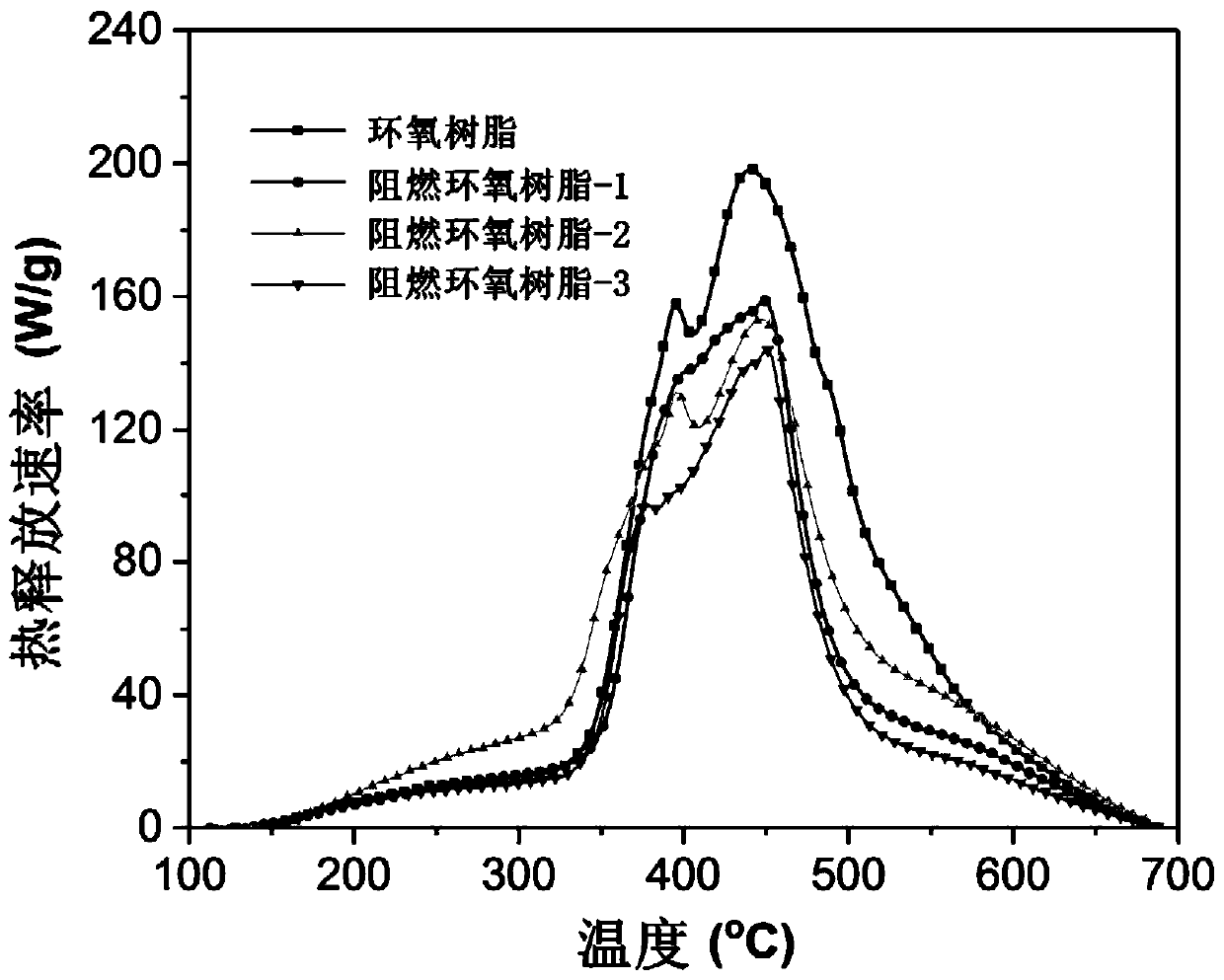
Image
Examples
Embodiment 1
[0028] ①React terephthalaldehyde and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide in a molar ratio of 1:2 under nitrogen protection in toluene solvent to reflux temperature After 3 hours, the solvent was removed by rotary evaporation to obtain a phosphorus-containing diol.
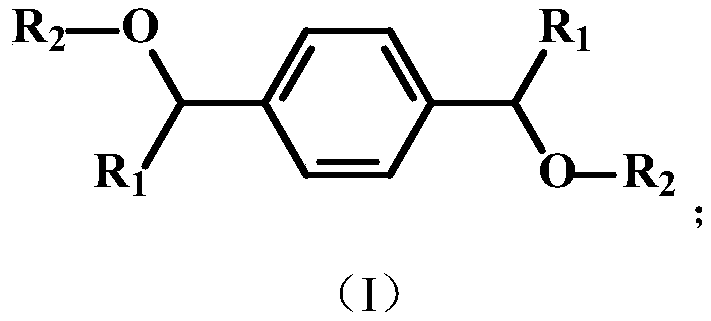
[0029] ②Heat the above phosphorus-containing dihydric alcohol, triethylamine, and diphenyl chlorophosphate to 60°C in toluene solvent according to the reaction molar ratio of 1:2:2, react for 24 hours, filter, and remove the solvent by rotary evaporation to obtain the target Product a, its chemical structure is as follows:
[0030]
[0031] The target product a was subjected to Fourier transform infrared spectroscopy (FT-IR), hydrogen nuclear magnetic resonance spectroscopy ( 1 H-NMR) characterization, confirmed that its chemical structure is as follows: FT-IR (KBr, cm -1 ): 754, 934 (P-O-C), 1226-1201 (P=O), 1589 (P-O-Ph). 1 H-NMR (400MHz, DMSO-d 6 ,ppm):5.40–5.13(m,2H,(P-CH-O) 2 ),8.27-8.13...
Embodiment 2
[0033] ① Heat terephthalaldehyde and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide in a molar ratio of 1:2.2 in toluene solvent to reflux temperature under the protection of argon After reacting for 6 hours, the solvent was removed by rotary evaporation to obtain phosphorus-containing dihydric alcohol.
[0034] ②Heat the above phosphorus-containing dihydric alcohol, triethylamine, and diphenylphosphinoyl chloride in toluene solvent to 100°C according to the reaction molar ratio of 1:2.2:2.2, react for 6 hours, filter, and remove the solvent by rotary evaporation to obtain the target Product b, its chemical structure is shown below:
[0035]
[0036] The target product b was subjected to Fourier transform infrared spectroscopy (FT-IR), hydrogen nuclear magnetic resonance spectroscopy ( 1 H-NMR) characterization, confirmed that its chemical structure is as follows: FT-IR (KBr, cm-1): 932 (P-O-C), 1192 (P=O), 1589 (P-Ar). 1 H-NMR (400MHz, DMSO-d 6 ,ppm):5.41–5.16(m,2H,...
Embodiment 3
[0038] ① Heat terephthalaldehyde and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide in a molar ratio of 1:2.1 in toluene solvent to reflux temperature under the protection of helium After reacting for 4 hours, the solvent was removed by rotary evaporation to obtain phosphorus-containing dihydric alcohol.
[0039] ②Heat the above-mentioned phosphorus-containing dihydric alcohol, triethylamine, and chlorinated diphenylphosphine to 80°C in toluene solvent according to the reaction molar ratio of 1:2.1:2.1, react for 12 hours, filter, and remove the solvent by rotary evaporation to obtain the target Product c, its chemical structure is shown below:
[0040]
[0041] The target product c was subjected to Fourier transform infrared spectroscopy (FT-IR), hydrogen nuclear magnetic resonance spectroscopy ( 1 H-NMR) characterization, confirmed that its chemical structure is as follows: FT-IR (KBr, cm -1 ):932(P-O-C),1231(P=O),1592cm -1 (P-Ar). 1 H-NMR (400MHz, DMSO-d 6 ,ppm)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com