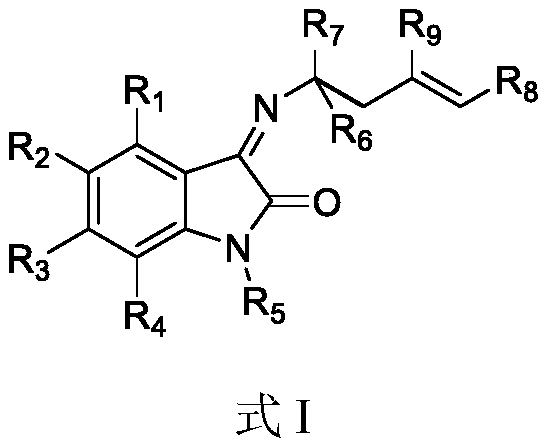
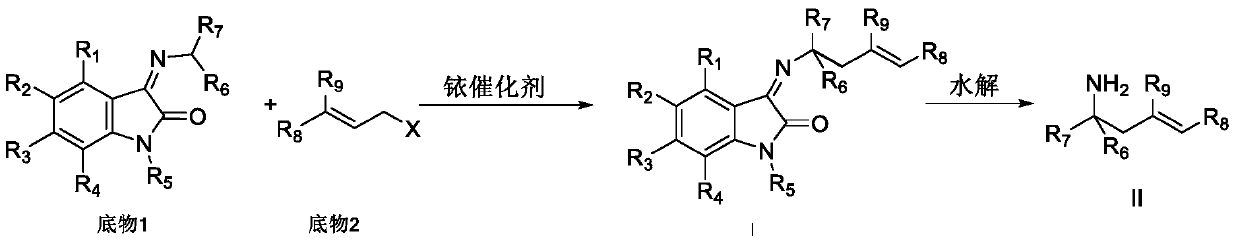
Chiral alpha-fluoroallamine derivative as well as preparation method and application thereof
A technology of high allylamine and derivatives, which is applied in the field of chemical medicine to achieve high yield, low catalyst consumption and good corresponding selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] preparation of
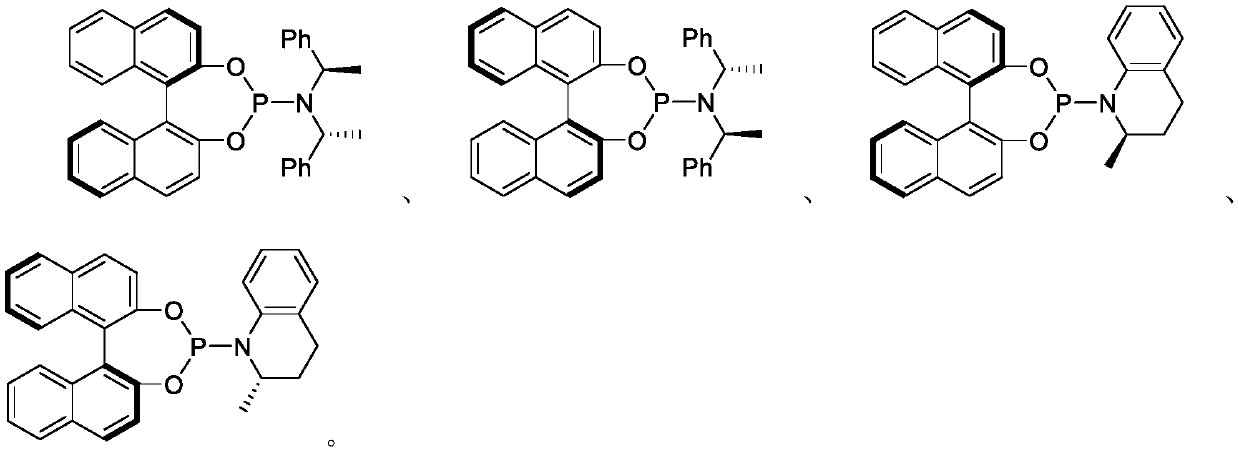
[0056] Add 0.005mmol[Ir(COD)Cl] to 25mL reaction tube 2 , 0.010mmol (S, S, S)-L1, 0.5mL deoxygenated THF and 0.5mL deoxygenated n-propylamine, reacted at 50°C for 30 minutes, and evaporated the solvent under reduced pressure to obtain an iridium catalyst. Under nitrogen protection at 25°C, 1 mL of dichloromethane was added, followed by 0.20 mmol of trifluoroethyl isatin imine and 0.22 mmol of cinnamyl methyl carbonate. After stirring for 24 h, the product was subjected to silica gel column chromatography (petroleum ether / ethyl acetate 20:1) to obtain a yellow liquid. Yield 99%, enantioselective excess of product 95%, HPLC (Chiralpak AS-H, i-propanol / hexane=5 / 95, flow rate 1.0mL / min, λ=254nm); t r =4.88and 5.29min); [α] 25 D =-90.0(c 0.18, CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl 3 )δ7.70(d, J=7.6Hz, 1H), 7.41(t, J=7.6Hz, 1H), 7.26–7.14(m, 5H), 7.09(t, J=7.6Hz, 1H), 6.70( d,J=7.6Hz,1H),6.23(d,J=16.0Hz,1H),6.17–6.05(m,2H),3.04–2.93(m,4H),2.64–2.53(m,...
Embodiment 2
[0058] preparation of
[0059] Add 0.005mmol[Ir(COD)Cl] to 25mL reaction tube 2 , 0.010mmol (S, S, S)-L1, 0.5mL deoxygenated THF and 0.5mL deoxygenated n-propylamine, reacted at 50°C for 30 minutes, and evaporated the solvent under reduced pressure to obtain an iridium catalyst. Under nitrogen protection at 25°C, 1 mL of dichloromethane was added, followed by 0.20 mmol of trifluoroethyl isatin imine and 0.22 mmol of p-methylphenylallyl methyl carbonate. After stirring for 24h, the product was subjected to silica gel column chromatography (petroleum ether / ethyl acetate 20:1) to obtain a yellow solid with a yield of 90%, a melting point of 80-82°C, and an enantioselective excess of 93% of the product, HPLC (Chiralpak AS-H, i-propanol / hexane=5 / 95, flow rate 1.0mL / min, λ=254nm; t r =4.62 and 4.83min.); [α] 25 D =-124.6(c 0.13, CH 2 Cl 2 ) 1 H NMR (400MHz, CDCl 3 )δ7.69(dd, J=7.6,0.8Hz,1H),7.41(td,J=7.9,1.2Hz,1H),7.13–6.98(m,5H),6.70(d,J=8.0Hz,1H ),6.21(d,J=15.6Hz,1H),6....
Embodiment 3
[0061] preparation of
[0062] Add 0.005mmol[Ir(COD)Cl] to 25mL reaction tube 2 , 0.010mmol (S, S, S)-L1, 0.5mL deoxygenated THF and 0.5mL deoxygenated n-propylamine, reacted at 50°C for 30 minutes, and evaporated the solvent under reduced pressure to obtain an iridium catalyst. Under nitrogen protection at 25°C, 1 mL of dichloromethane was added, followed by 0.20 mmol of trifluoroethyl isatinimine and 0.22 mmol of p-methoxyphenylallyl methyl carbonate. After stirring for 24h, the product was subjected to silica gel column chromatography (petroleum ether / ethyl acetate 20:1) to obtain a yellow liquid with a yield of 92% and an enantioselective excess of 94%. Chiralpak AS-H, i-propanol / Hexane=5 / 95, flow rate 1.0mL / min, λ=254nm, t r =6.48and 6.78min.)[α] 30 D =-81.0(c 0.20, CH 2 Cl 2 ); 1 H NMR (400 MHz, CDCl 3 )δ7.69(d, J=7.2Hz, 1H), 7.41(td, J=8.0, 1.2Hz, 1H), 7.15–7.05(m, 3H), 6.77(d, J=8.8Hz, 2H), 6.70(d,J=7.6Hz,1H),6.16(d,J=16.0Hz,1H),6.11–6.01(m,1H),6.01–5.88(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com