Peroxy bond-containing compound Oleracone I in parslane herb as well as extraction and separation method and application of compound
A separation method and compound technology, which can be applied in the directions of organic chemistry, drug combination, non-central analgesics, etc., can solve the problems of low structural novelty, and achieve the effects of environmental protection of the process method, simple and fast operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention provides a new compound with the molecular formula C 18 H 18 O 6 , Named oleracone I, chemical formula:
[0031] .
[0032] The new compound is named oleracone I according to the structure. Table 1 shows the nuclear magnetic data of the new compound: 1 H-NMR and 13 C-NMR in deuterated DMSO.
[0033] Table 1 NMR data of new compounds ( 1 H-NMR and 13 C-NMR in deuterated DMSO).
[0034]
[0035] The structure identification of the compound of the present invention Figure 1-10 .
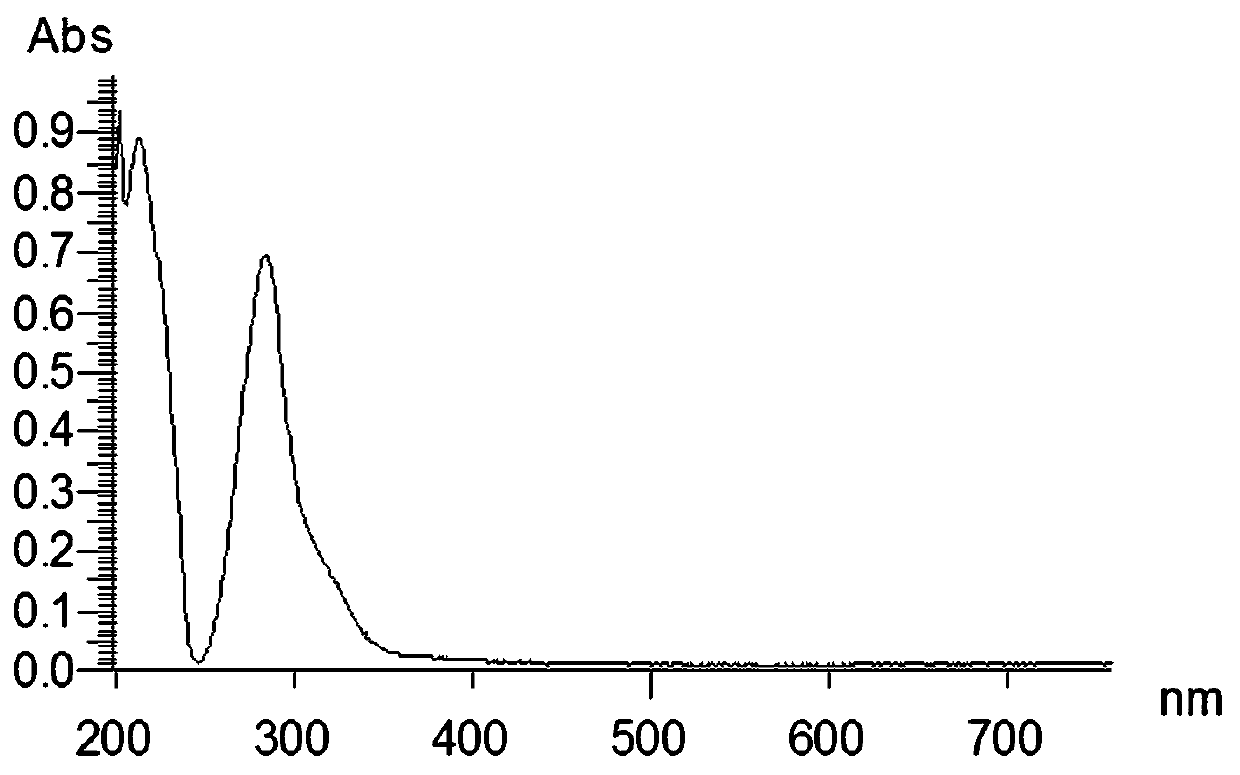
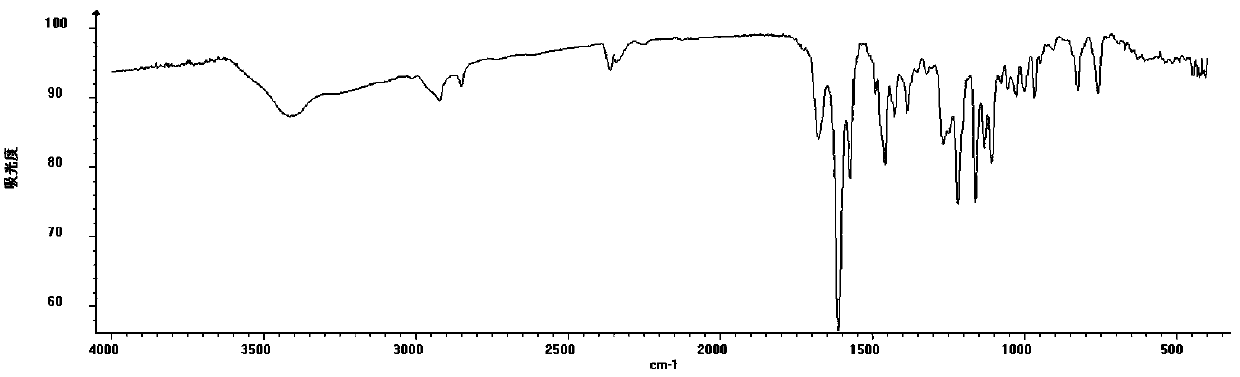
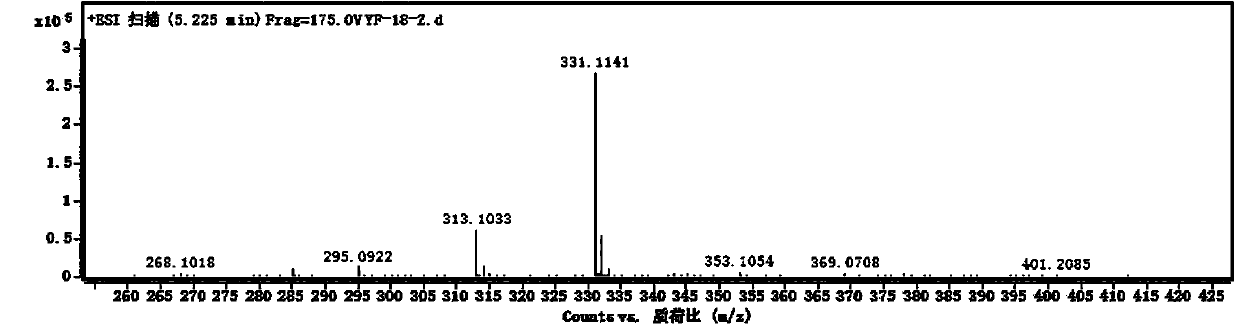
[0036] Oleracone I: yellow-brown powder, easily soluble in methanol. UV(MeOH)λ max : 284, 214 nm. IR (KBr) v max 2920, 2850, 2360, 1670, 1610, 1460, 1215, 1160, 831, 762 cm -1 . HRESI(+)TOFMS gives m / z: 331.1141 [M + H] + The quasi-molecular ion peak has a molecular weight of 331.1137. Combine 1 H-NMR, 13 C-NMR and DEPT data, speculate that the possible molecular formula of the compound is C 18 H 18 O 6 , The degree of unsaturation is 10. 13 C-NMR spectrum and DEPT spectrum s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com