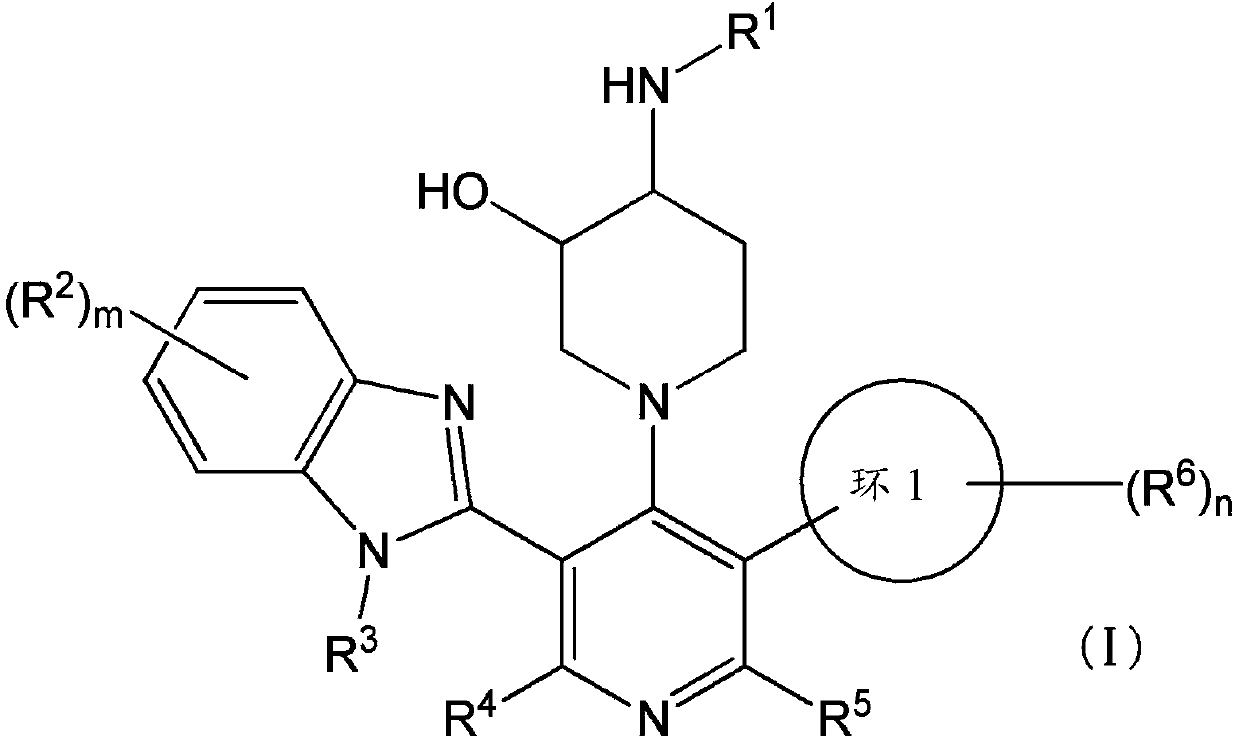
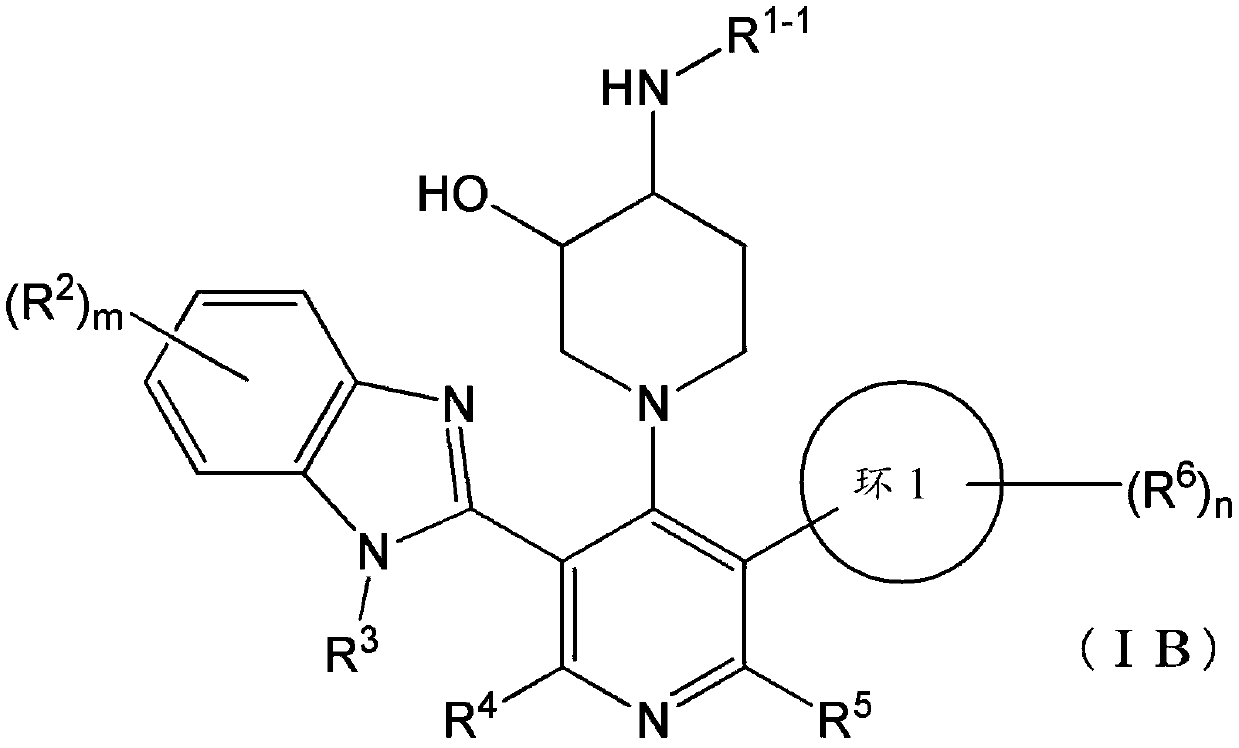
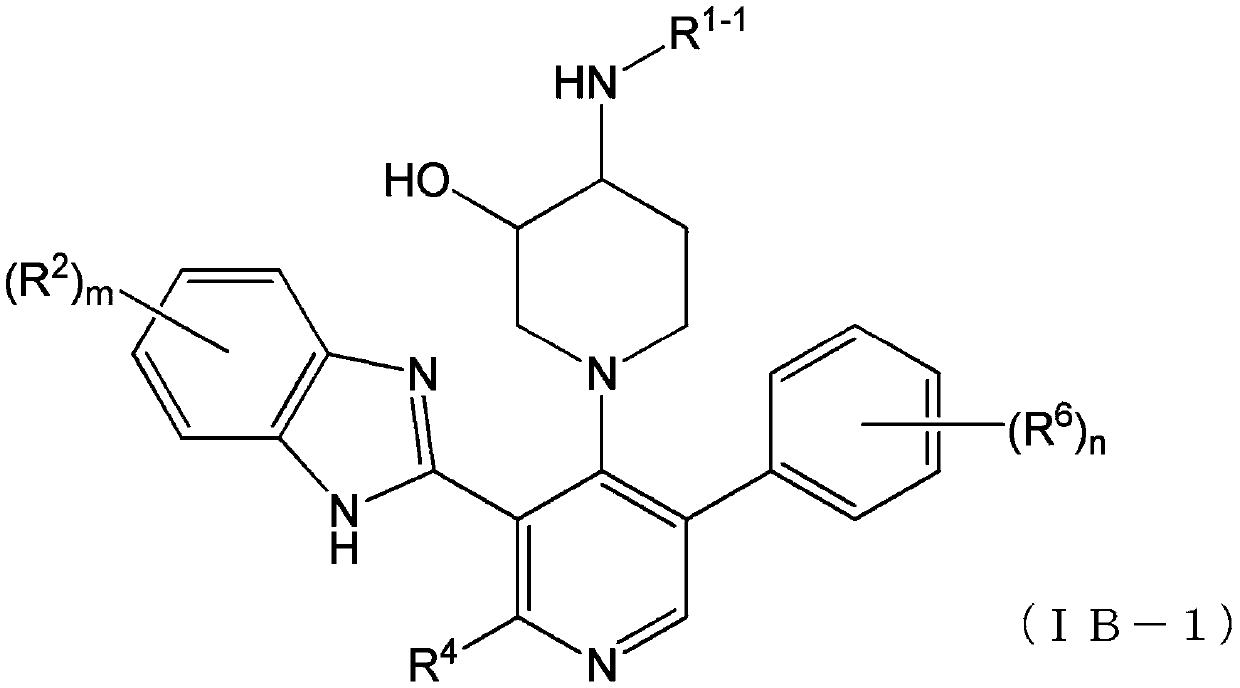
Compound having somatostatin receptor agonistic activity and pharmaceutical use thereof
A kind of compound and halogen technology, applied in the compound with somatostatin receptor agonistic activity and the field of medical use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0542] 2-{4-[(3S,4R)-4-amino-3-hydroxy-1-piperidinyl]-5-(3,5-difluorophenyl)-2-(1-methyl-1H- Pyrazol-4-yl)-3-pyridyl}-1H-benzimidazole-7-carboxylic acid methyl ester
[0543] [C34]
[0544]
[0545] To a solution of the compound prepared in Reference Example 5 in dichloromethane (1.0 mL) was added trifluoroacetic acid (87 µL) under a nitrogen atmosphere, and the mixture was stirred at room temperature for 3 hr. The reaction solution was concentrated under reduced pressure, and the resulting residue was purified by medium-pressure preparative liquid chromatography (Hi-flash NH) (ethyl acetate:methanol=100:0 to 80:20) to obtain the present compound (7 mg ).
[0546] HPLC retention time (min): 0.57;
[0547] MS (ESI, Positive): 560 (M+H) + ;
[0548] 1 H-NMR (CD 3 OD): δ 8.38, 8.04, 8.02, 7.51-7.41, 7.28-7.18, 7.08-6.98, 6.85, 3.96, 3.72, 3.37-3.28, 3.21-3.09, 2.93-2.73, 2.46-2.37, 2.23-2.17, 1.09- 0.79.
[0549] Embodiment 1(1) to 1(4)
[0550] The compound of ...
Embodiment 2
[0578] (3S,4R)-4-amino-1-[5-(3-chloro-5-fluorophenyl)-3-(6-methoxy-1H-benzimidazol-2-yl)-2- Methyl-4-pyridyl]-3-piperidinol
[0579] [C35]
[0580]
[0581] The compound of the present invention having the following properties was prepared in a similar manner to that in Reference Example 4→Reference Example 5→Example 1, wherein the compound prepared in Reference Example 6 was used instead of the compound prepared in Reference Example 3, 3- Chloro-5-fluorophenylboronic acid instead of 3,5-difluorophenylboronic acid, 4-methoxybenzene-1,2-diamine instead of methyl 2,3-diaminobenzoate.
[0582] HPLC retention time (min): 0.52;
[0583] MS (ESI, positive): 482 (M+H) + ;
[0584] 1 H-NMR (CD 3 OD): δ 8.21, 7.58-7.42, 7.37-7.20, 7.18-7.09, 7.00-6.90, 3.87, 3.43-3.36, 3.11-2.98, 2.91-2.70, 2.50-2.39, 2.36, 2.19-2.07, 1.12-0.95.
[0585] Embodiment 2(1) to 2(13)
[0586] The compound of the present invention having the following properties is prepared in the same steps ...
Embodiment 3
[0656] (3S,4R)-1-[5-(3-chloro-5-fluorophenyl)-3-(6-methoxy-1H-benzimidazol-2-yl)-2-methyl-4- Pyridyl]-4-(3-oxetanylamino)-3-piperidinol
[0657] [C37]
[0658]
[0659] Under a nitrogen atmosphere, acetic acid (30 μL) was added to a suspension of the compound prepared in Example 2 (48 mg) in methanol (1.0 mL), to which 3-oxetanone (58 μL) and 2-picoline were added Borane complex (19.3 mg), and the mixture was stirred at room temperature. After 13 hours, 3-oxetanone (39 μL) and 2-picoline borane complex (13.9 mg) were further added, and the mixture was stirred at room temperature for 13 hours. To the reaction solution was added saturated aqueous sodium bicarbonate solution, followed by extraction with ethyl acetate, and the organic layer was concentrated under reduced pressure. The resulting residue was purified by medium-pressure preparative liquid chromatography (Hi-flash NH) (ethyl acetate:methanol=100:5 to 80:20) to obtain the present compound (45 mg) having the fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com