Compound containing naphthoquinone oxime structure and medical application of compound as bombesin receptor subtype 3 agonist
A technology of a compound, naphthoquinone oxime, applied in the field of medicinal chemistry, can solve problems such as poor selectivity and defects, and achieve the effects of simple preparation method, strong agonist activity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
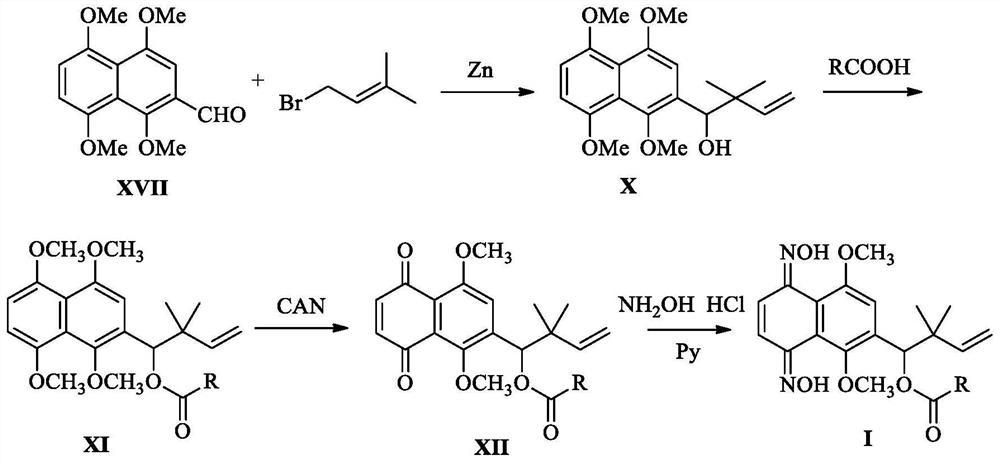
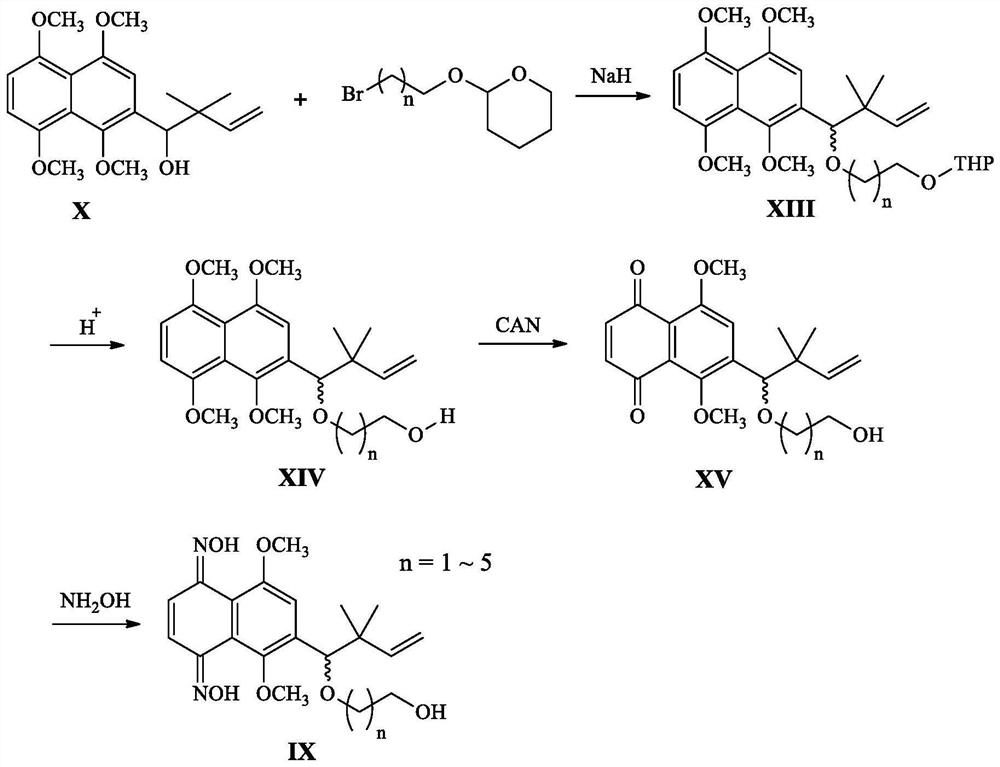
[0061] This embodiment relates to a kind of 6-(4-hydroxyl-3,3-dimethyl-1-enebutyl)-5,8-dimethoxy-1,4-naphthalene with structural formula (Ⅲ-1) Synthesis of diketodioxime furan-2-carboxylate, the synthetic route is attached figure 1 As shown, it specifically includes the following steps:
[0062] Step 1. Synthesis of intermediate 2-(4-hydroxyl-3,3-dimethyl-1-enyl)-1,4,5,8-tetramethoxynaphthalene with structural formula (X):
[0063]
[0064] Specifically include the following steps:
[0065] Zinc powder (10 equivalents) and bromoisoamylene (5 equivalents) were added to 1,4,5,8-tetramethoxy-2-naphthaldehyde (1.38 g, figure 1 Formula (XVII) shown in, the molar ratio of formula (XVII) and bromoisoamylene is 1:5) in tetrahydrofuran solution (20mL), then slowly add saturated aqueous ammonium chloride solution (10mL) at 0°C , TLC monitored the reaction. After Compound IX disappeared, the zinc powder was removed by filtration, and ethyl acetate (50 mL) and 2.0 N HCl (25 mL) wer...
Embodiment 2
[0079]
[0080] This embodiment relates to a kind of 6-(4-hydroxyl-3,3-dimethyl-1-enebutyl)-5,8-dimethoxy-1,4-naphthalene with structural formula (IV-1) The synthesis of diketodioxime tetrahydrofuran-2-formate, the synthetic route is attached figure 1 As shown, the specific steps are basically the same as in Example 1, except that in Step 2, this example uses tetrahydrofuran-2-carboxylic acid instead of furan-2-carboxylic acid.
[0081] The obtained product was light yellow powder with a total yield of 36.1%. 1 H NMR (400MHz, DMSO-d 6 ):δ12.13(s,1H),12.08(s,1H),7.39(dd,J=1.3Hz,2H),6.87(d,J=3.1Hz,1H),6.05–5.92(m,2H) ,5.04(dt,J=10.8,1.3Hz,1H),4.95(dd,J=17.6,1.4Hz,1H),4.59–4.48(m,1H),3.84(td,J=6.7,2.4Hz,2H ),3.75(s,2H),3.67(s,3H),2.33–2.17(m,1H),2.02–1.79(m,3H),1.05(d,J=3.1Hz,3H),1.00(s, 3H).
Embodiment 3
[0083]
[0084]
[0085] This embodiment relates to a kind of 6-(4-hydroxyl-3,3-dimethyl-1-enebutyl)-5,8-dimethoxy-1,4-naphthalene with structural formula (V-1) The synthesis of diketodioxime pyridine-2-carboxylate, the synthetic route is attached figure 1 As shown, the specific steps are basically the same as in Example 1, except that in Step 2, this example uses pyridine-2-carboxylic acid instead of furan-2-carboxylic acid.
[0086] The obtained product was a light yellow powder with a total yield of 37.2%. 1 H NMR (400MHz, DMSO-d 6 )δ12.17(s,1H),12.09(s,1H),8.78(d,J=4.8Hz,1H),8.18(d,J=7.8Hz,1H),8.04(td,J=7.8,1.8 Hz,1H),7.67(dd,J=7.6,4.8Hz,1H),7.39(s,2H),7.03(s,1H),6.25(s,1H),6.13(dd,J=17.5,10.8Hz ,1H),5.06(d,J=10.8Hz,1H),4.99(d,J=17.5Hz,1H),3.75(s,3H),3.68(s,3H),1.12(d,J=16.9Hz ,7H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com