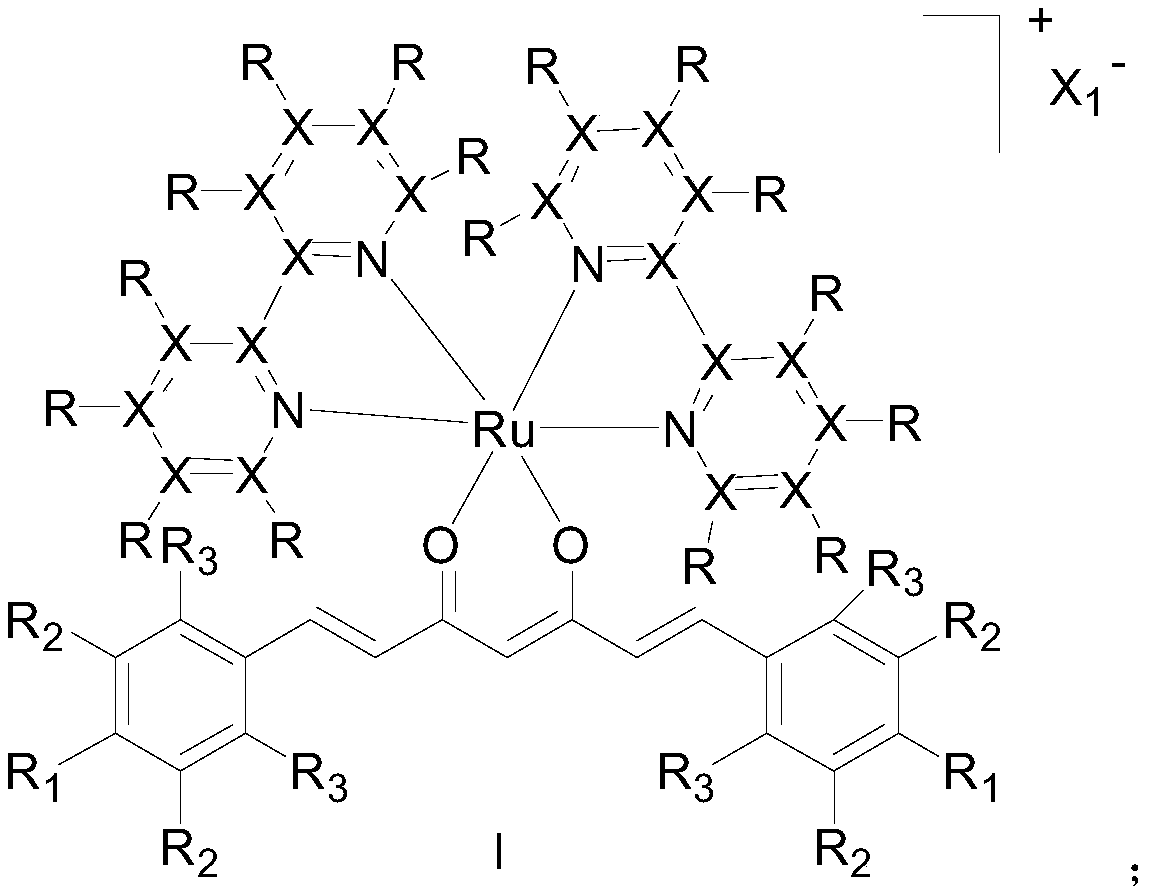
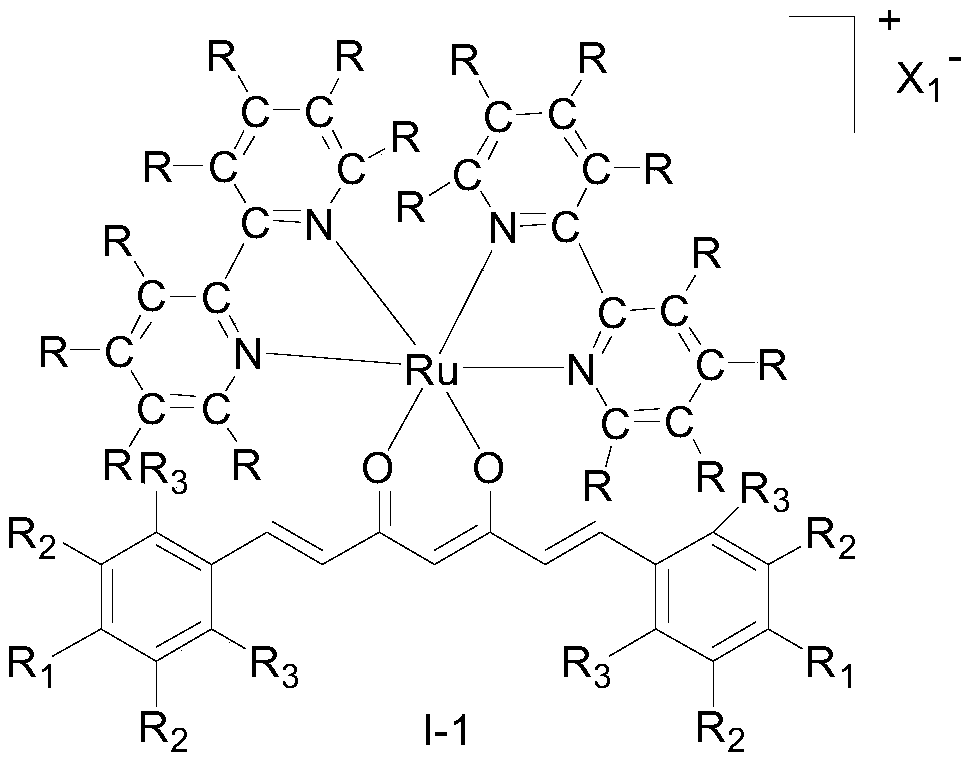
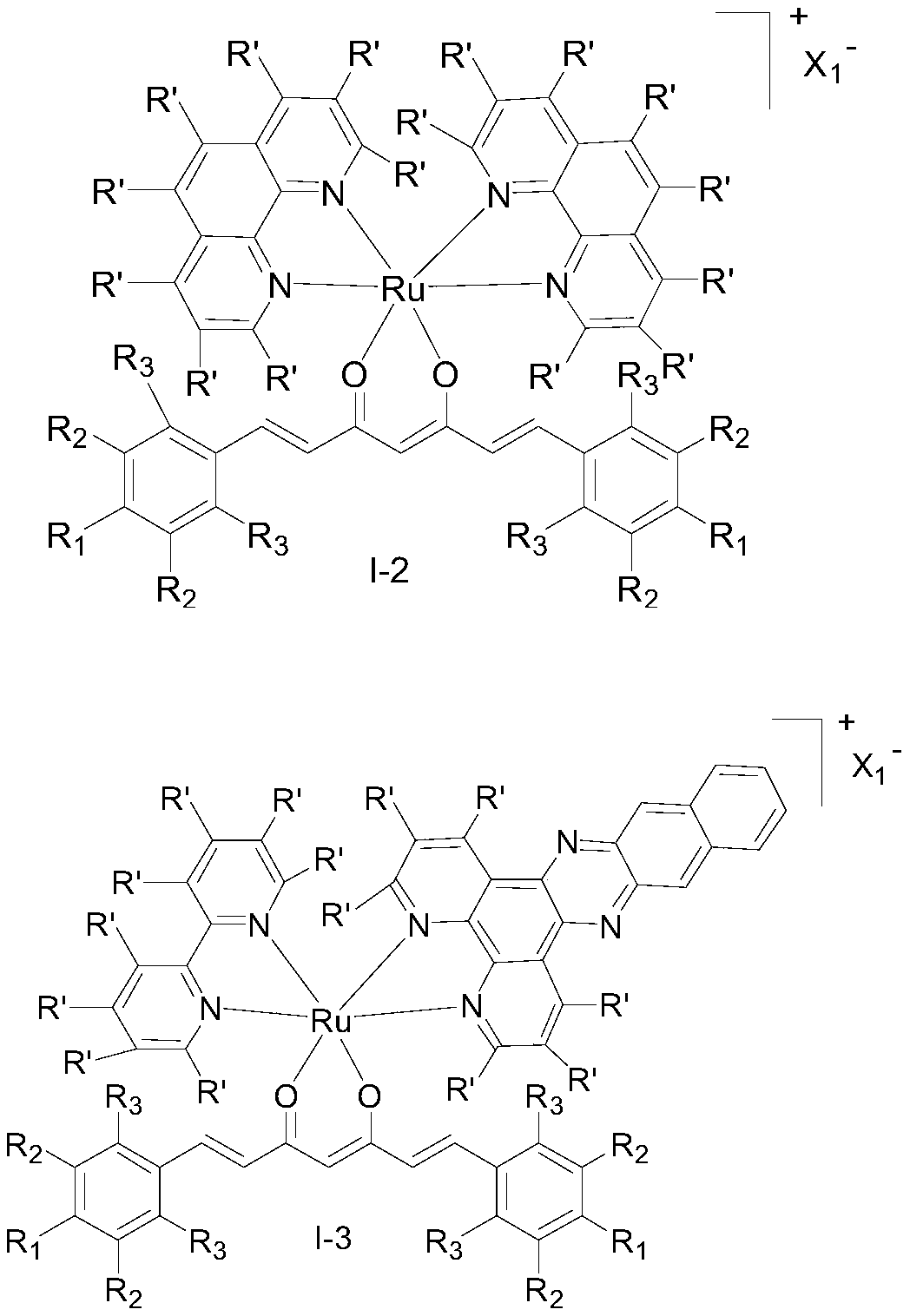
Ruthenium (II) complex with curcumin derivative as ligand, and preparation method and application thereof
A technology of curcumin derivatives and complexes, applied in ruthenium organic compounds, compounds containing group 8/9/10/18 elements of the periodic table, drug combinations, etc., can solve low cell uptake and tissue distribution, and clinical application Obstacles, instability and other problems, to achieve the effect of improving hydrolysis stability, simple and convenient preparation method, and good inhibition of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of complex 1:
[0054] Cis-(bpy) 2 RuCl 2 (48.40mg, 0.10mmol), curcumin (368.4mg, 1.0mmol), LiCl (42.0mg, 1.0mmol) in EtOH / H 2 O (30 / 10ml) was mixed, stirred and refluxed for 12h to obtain a black solution, the solvent was removed, and column chromatography DCM:MeOH=10:1 gave a dark brown powder.
[0055] Yield: 52.0%. Black-brown powder. Anal. Calcd (%) for C 41 h 35 ClN 4 o 6 Ru: C 60.33, H 4.32, N 6.86. Found: C 60.18, H 4.37, N 6.97; ESI-MS: m / z [M-Cl] + =781.16; 1 HNMR (600MHz, DMSO-d 6 )δ3.75(s,6H),5.96(s,1H),6.58-6.61(d,2H,J=15.8Hz),6.79-6.81(d,2H,J=8.2Hz),6.88-6.90(m ,2H),6.95-6.97(d,2H,J=15.7Hz),7.11-7.12(m,2H),7.29-7.32(t,2H,J=6.6Hz),7.76-7.80(m,4H), 7.91-7.93(t, 2H, J=7.6Hz), 8.16-8.19(t, 2H, J=7.6Hz), 8.64-8.65(d, 2H, J=5.3Hz), 8.74-8.76(d, 2H, J=8.0Hz), 8.85-8.86(d, 2H, J=8.1Hz); 13 C NMR (150MHz, DMSO-d 6 )δ56.02,101.87,110.68,116.08,122.42,123.97,124.02,126.26,126.39,126.93,127.31,135.44,136.47,137.04,148.39,148.77,149.81,153.23,15...
Embodiment 2
[0057] Synthesis of complex 2:
[0058] Cis-(phen) 2 RuCl 2 (53.2mg, 0.10mmol), curcumin (368.4mg, 1.0mmol), LiCl (42.0mg, 1.0mmol) in EtOH / H 2 O (30 / 10ml) was mixed, stirred and refluxed for 12h to obtain a black solution, the solvent was removed, and column chromatography DCM:MeOH=15:1 gave a dark brown powder.
[0059] Yield: 48.6%. Black-brown powder. Anal. Calcd (%) for C 45 h 35 ClN 4 o 6 Ru: C 62.53, H 4.08, N 6.48. Found: C 62.38, H 4.27, N 6.69; ESI-MS: m / z [M-Cl] + =829.16; 1 HNMR (600MHz, DMSO-d 6 )δ3.73(s,6H),6.05(s,1H),6.58-6.61(d,2H,J=15.9Hz),6.77-6.79(d,2H,J=8.2Hz),6.85-6.86(m ,2H),6.93-6.95(d,2H,J=15.8Hz),7.07(m,2H),7.53-7.55(m,2H),8.01-8.02(d,2H,J=5.1Hz),8.20- 8.22(m,2H),8.28-8.30(d,2H,J=8.8Hz),8.37-8.38(d,2H,J=8.9Hz),8.49-8.50(d,2H,J=8.0Hz),8.84 -8.85(d, 2H, J=8.1Hz), 9.12-9.13(d, 2H, J=5.0Hz), 9.66(m, 2H); 13 CNMR (150MHz, DMSO-d 6 )δ56.03,100.00,101.76,110.81,116.08,122.33,125.34,126.02,126.34,127.31,128.07,128.15,130.30,130.45,134.38,136.06,13...
Embodiment 3
[0061] Synthesis of complex 3:
[0062] [Ru(bpy)(dppn)Cl 2 ] (66.1mg, 0.1mmol), curcumin (368.4mg, 1.0mmol), LiCl (42.0mg, 1.0mmol) in EtOH / H 2 O (30 / 10ml) was mixed, stirred and refluxed for 12h to obtain a black solution, the solvent was removed, and column chromatography DCM:MeOH=15:1 gave a dark brown powder. Yield: 40.5%. Black-brown powder. Anal. Calcd (%) for C 53 h 39 ClN 6 o 6 Ru: C 64.14, H 3.96, N 8.47. Found: C 63.98, H 4.13, N 8.69; ESI-MS: m / z [M-Cl] + =957.21; 1 H NMR (600MHz, DMSO-d 6 )δ3.54(s,3H),3.79(s,3H),6.09(s,1H),6.60-6.61(d,1H,J=8.3Hz),6.69-6.71(d,2H,J=15.9Hz ),6.78-6.82(m,2H),6.94-6.95(m,1H),6.99(m,1H),7.06-7.09(d,1H,J=15.8Hz),7.15-7.17(m,2H), 7.21-7.23(t,1H,J=6.6Hz),7.63(m,2H),7.71-7.72(m,1H),7.83-7.84(d,1H,J=5.6Hz),7.88-7.93(m, 2H),8.09-8.10(m,1H),8.23-8.29(m,4H),8.75-8.77(d,1H,J=8.2Hz),8.84-8.90(m,3H),8.96(m,1H) ,9.03-9.04(d,2H,J=5.2Hz),9.26(m,1H),9.43(s,1H),9.57(s,1H); 13 C NMR(150MHz,DMSO-d6)δ55.70,56.10,102.66,110.86,116.06,116.19,122....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com