Novel peptidome for improving pre-eclampsia placental dysfunction
A technology for preeclampsia and dysfunction, applied in the field of medicine, can solve the problems of maternal-fetal toxicity and side effects, and achieve the effects of good stability, low molecular weight and good lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
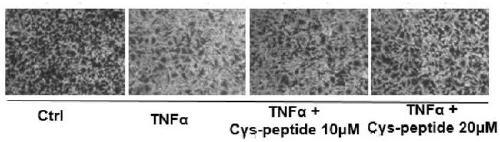
[0025] Further, the application of the polypeptide in a medicine or medicament for improving placental dysfunction of preeclampsia, wherein the polypeptide and the polypeptide composition can be made into any pharmaceutically acceptable dosage form.
[0026] The rats are bred in an environment with controlled light and humidity, eat freely and drink tap water freely. Under these conditions, they can adapt for a week. Fertile females and males are reared at a ratio of 1:1 overnight, and the plugs are picked in the morning, such as Existence is confirmed to have been pregnant, this day is GD 0. At the 14th day of pregnancy, pregnant mice were randomly assigned to three groups. The first group (Saline) (n=10): On the 14th day of pregnancy, each rat received normal saline (1mg / kg) through the tail vein; the second group (LPS): On the 14th day of pregnancy, LPS was injected through the tail vein (1μg / kg); The third group (LPS+Cys-peptide): At the 14th day of pregnancy, the rats were ...
Embodiment 2
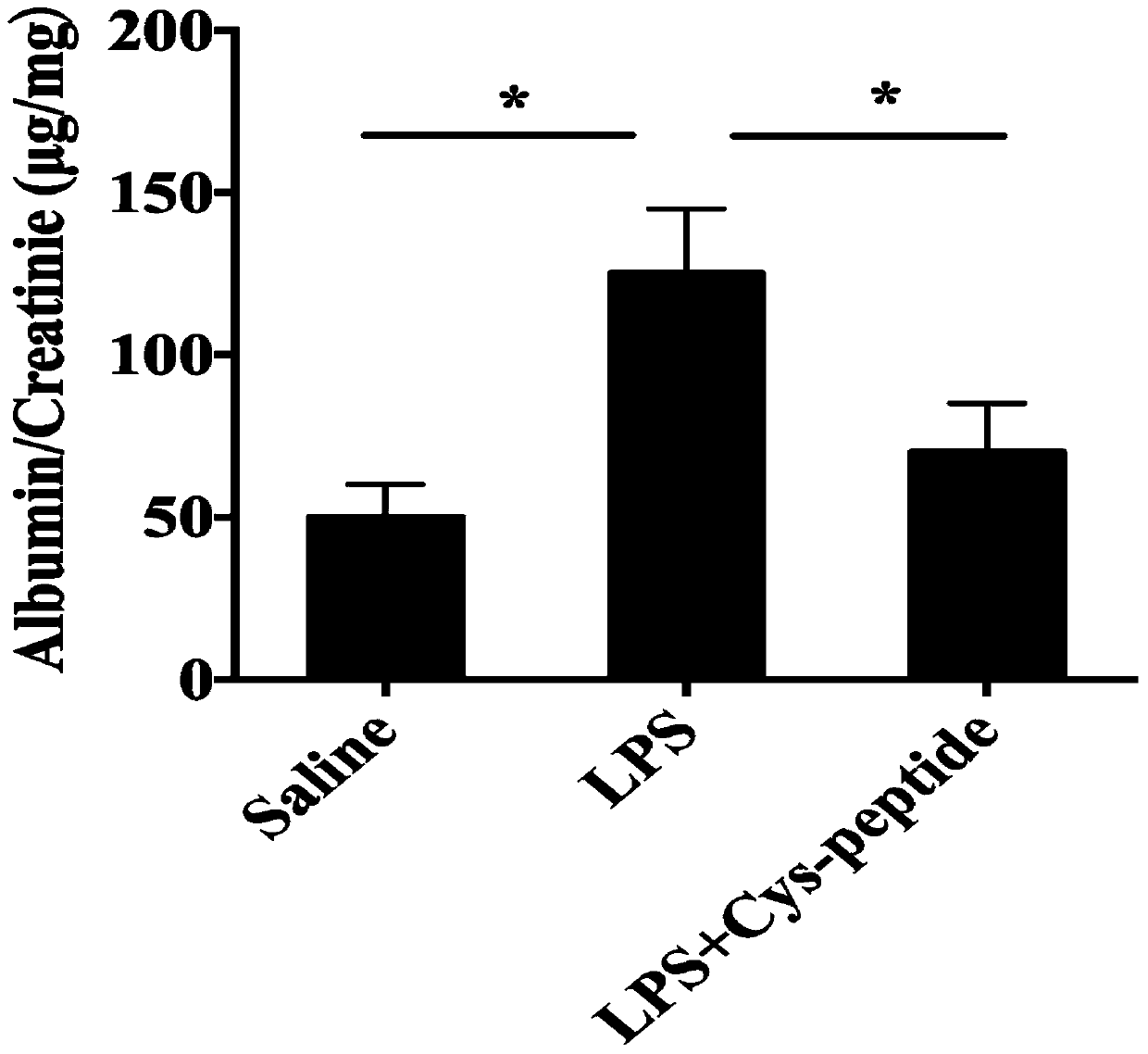
[0033] Further, the application of polypeptides in reducing urine protein / creatinine levels in preeclampsia pregnant rats.
[0034] Sprague-Dawley (SD) rats (10-12 weeks, 280-350g) were purchased from the Experimental Animal Center of Nanjing Medical University. The rats are kept in an environment with controlled light and humidity, eat freely and drink tap water freely.
[0035] After one week of acclimatization, the fertile females and males were reared overnight at a ratio of 1:1, and the plugs were picked up in the morning. If they exist, they were confirmed to be pregnant. This day is GD 0. (Under standard laboratory conditions (temperature 23-26℃, relative humidity 50-60%, irradiation between 06:00am and 06:00pm), free access to pelleted food and water)
[0036] At the 14th day of pregnancy, pregnant rats were randomly assigned to three groups: Group 1 (Sal ine) (n=10): On day 14 of pregnancy, each rat received normal saline (1mg / kg) through the tail vein; Group 2 (PE ) Rats ...
Embodiment 3
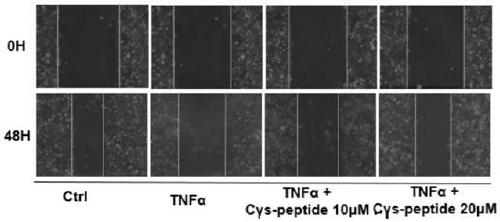
[0041] Further, the application of the polypeptide in improving the utilization rate of the placenta of pregnant mice with preeclampsia.
[0042] Praague-Dawley (SD) rats (10-12 weeks, 280-350g) were purchased from the Experimental Animal Center of Nanjing Medical University. The rats are kept in an environment with controlled light and humidity, eat freely and drink tap water freely.
[0043] After one week of acclimatization, the fertile females and males were reared overnight at a ratio of 1:1, and the plugs were picked up in the morning. If they exist, they were confirmed to be pregnant. This day is GD 0. (Under standard laboratory conditions (temperature 23-26℃, relative humidity 50-60%, irradiation between 06:00am and 06:00pm), free access to pelleted food and water)
[0044] At the 14th day of pregnancy, pregnant rats were randomly assigned to three groups: Group 1 (Sal ine) (n=10): On day 14 of pregnancy, each rat received normal saline (1mg / kg) through the tail vein; Group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com