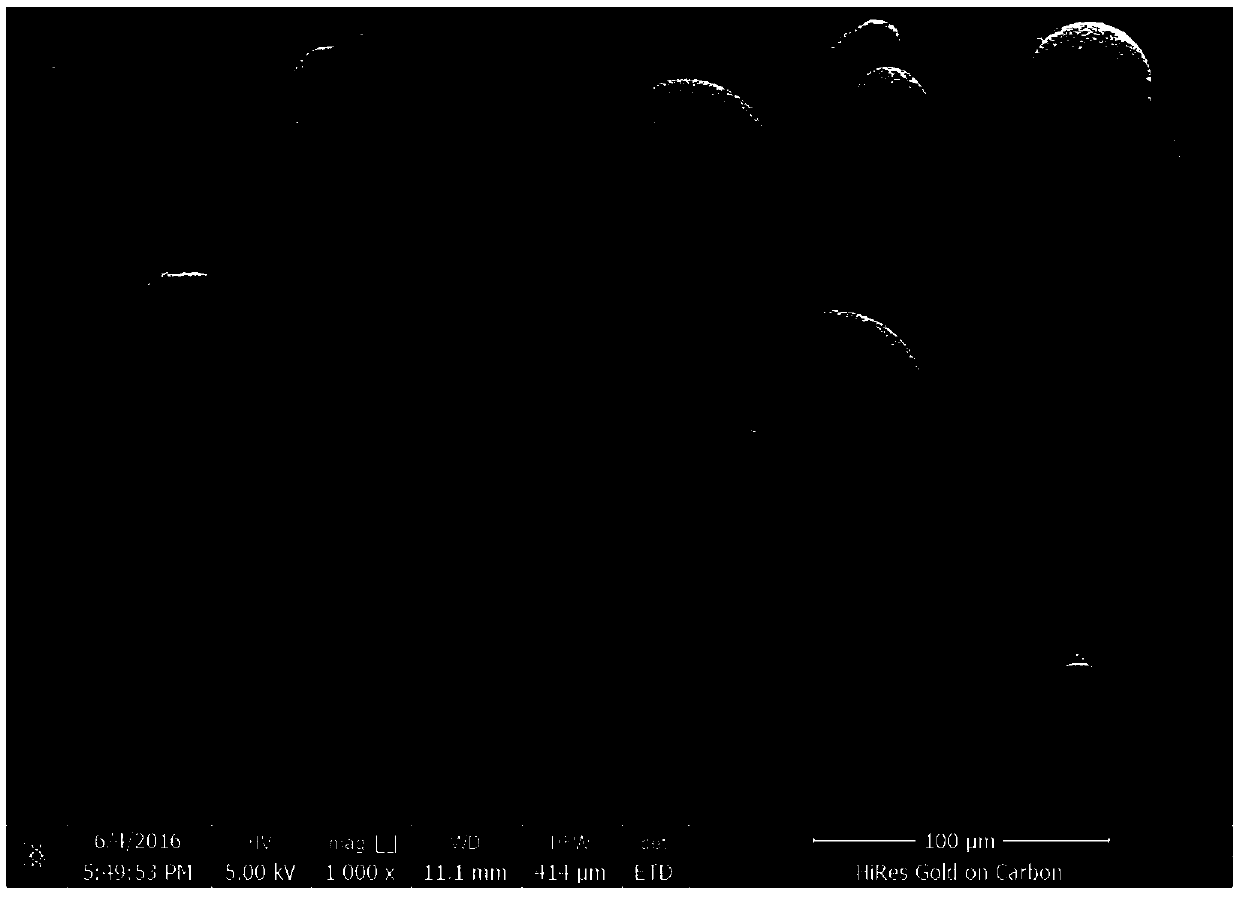
Traditional Chinese medicine embolization microspheres used for interventional treatment of liver cancer at middle and advanced stages and preparation method of traditional Chinese medicine embolization microspheres
A technology of interventional therapy and embolization of microspheres, which is applied in the field of medicine, can solve the problems of drug resistance of tumor cells, easy occurrence of collateral circulation, and difficulty in entering tumor cells, etc., so as to inhibit the occurrence of drug resistance, have low toxic and side effects, and improve life quality. quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of traditional Chinese medicine embolization microspheres for interventional treatment of advanced liver cancer is characterized in that it comprises the following steps of specific preparation:
[0038] Step 1: Weigh 75.48% to 81.99% of Baiji polysaccharide, 0.5% to 1.5% of sodium cantharidate, 2.5 to 3.0% of lecithin, 0.01 to 0.02% of cholesterol and 15% to 20% of 1,2-propanediol according to the mass percentage , the sum of the mass percentages of the above components is 100%;
[0039] Step 2: Add the 1,2-propanediol and sodium cantharidate weighed in step 1 into water for injection, dissolve to obtain a sodium cantharidate solution, and keep it warm at 50°C; add the lecithin and cholesterol weighed in step 1 Shake and dissolve with absolute ethanol for injection, then put it into the sodium cantharidinate solution, and stir for 15-20min under the protection of nitrogen atmosphere of 1.5-1.8MPa, the stirring speed is 1300-1500r·min -1 , and th...
Embodiment 1
[0055] Step 1: Weigh 81.99% (81.99g) of Baiji polysaccharide, 0.5% (0.5g) of sodium cantharidinate, 2.5% (2.5g) of lecithin, 0.01% (0.01g) of cholesterol and 1,2- Propylene glycol 15% (15mL), the sum of the mass percentages of the above components is 100%.
[0056] The lecithin, cholesterol and 1,2-propanediol mentioned above are all injection-grade pharmaceutical excipients.
[0057] Step 2: Add 0.5g of sodium cantharidate and 15mL of 1,2-propanediol for injection into 50mL of water for injection weighed in step 1, dissolve to obtain sodium cantharidate solution, and keep warm at 50°C; weigh 2.5g Lecithin for injection and 0.01g of cholesterol for injection were added to 10mL of absolute ethanol for injection and shaken to dissolve, then injected into the sodium cantharidate solution obtained above, and stirred for 15min under the protection of a nitrogen atmosphere of 1.5MPa, stirring The speed is 1300r·min -1 , and then circulated and filtered through a 0.22 μm microporou...
Embodiment 2
[0062] Step 1: Take by weighing 75.48% Baiji polysaccharide (75.48g), sodium cantharidate 1.5% (15g), lecithin 3.0% (3.0g), cholesterol 0.02% (0.02g) and 1,2- Propylene glycol 20% (20mL), the sum of the mass percentages of the above components is 100%.
[0063] The lecithin, cholesterol and 1,2-propanediol mentioned above are all injection-grade pharmaceutical excipients.
[0064] Step 2: Add 1.5g of sodium cantharidate and 20mL of 1,2-propylene glycol for injection weighed in step 1 into 45mL of water for injection, dissolve to obtain a sodium cantharidate solution, and keep warm at 50°C; weigh step 1 Add 3.0g of lecithin for injection and 0.02g of cholesterol for injection into 10mL of absolute ethanol for injection and shake to dissolve, then inject it into the sodium cantharidate solution obtained above, and stir under the protection of a nitrogen atmosphere of 1.6MPa 20min, the stirring rate is 1500r·min -1 , and then circulated and filtered through a 0.22 μm microporous ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com