Eutectic compound formed by lipase inhibitor and hydroxy methylglutaryl coenzyme A reductase inhibitor
A technology of hydroxymethylglutaryl coenzyme and lipase inhibitor, which is applied in the field of co-crystal complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] 1 Preparation of eutectic composite
[0057] The present invention prepares the eutectic composite described in the present invention with reference to the grinding method disclosed by Scott C. McKellar et al. (Crystal Growth & Design 2014, 14, 5, 2422-2430).
[0058] Specifically, a certain molar ratio of the lipase inhibitor and the hydroxymethylglutaryl-CoA reductase inhibitor is placed in a ball mill, and ground at a frequency of 30-60 Hz for 15-60 minutes at room temperature. The frequency and grinding time of the ball mill were optimized and screened with the product melting distance ≤ 2°C as the standard.
[0059] 2 Structure confirmation and characterization of the eutectic complex
[0060] 2.1 Determination of eutectic formation and preliminary detection of purity
[0061] A single eutectic complex was considered to have formed if the milled product had a melting range below 2°C.
[0062] 2.2 Determination of the molar ratio of lipase inhibitor to hydroxymet...
Embodiment 1
[0070] Example 1. Preparation of orlistat·atorvastatin co-crystal complex
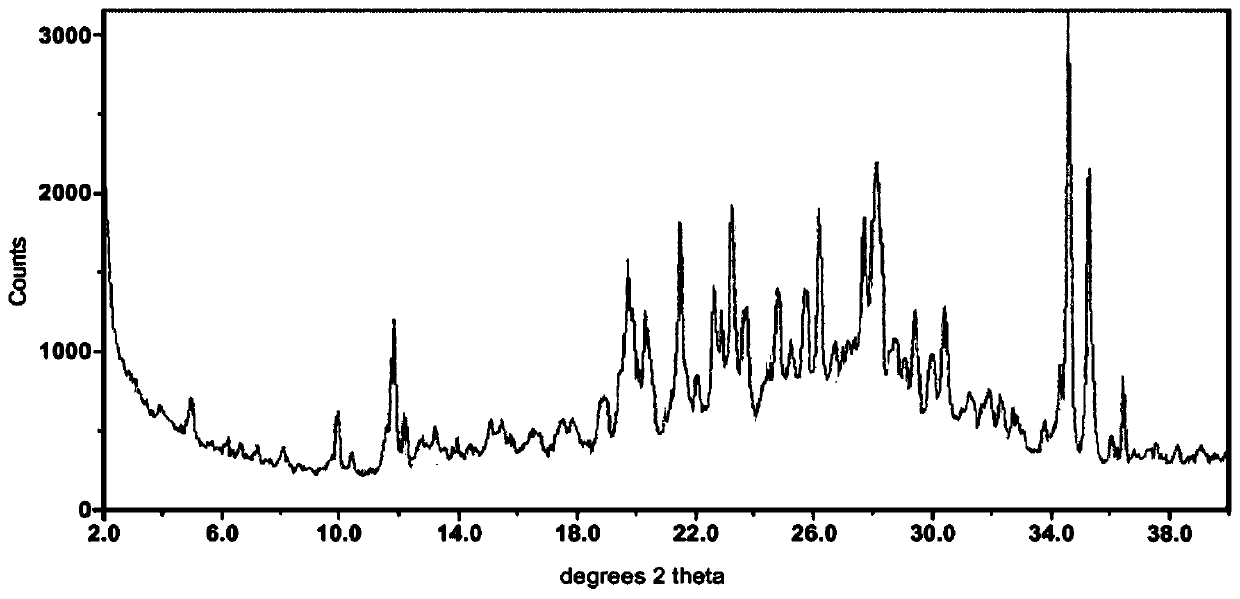
[0071] Take 148.719g of orlistat and 55.864g of atorvastatin, mix them thoroughly, place them in a planetary ball mill, grind them for 55min at a speed of 350r / min, and collect the product to obtain 192.915g of off-white powder with a melting point of 73.3-75.3°C . After recrystallization with ethanol, 192.306g of white crystalline powder was obtained, the melting point was 73.4-74.3°C, the R value was 2.858, and its X-ray powder diffraction pattern was as follows figure 1 shown.
Embodiment 2
[0072] Example 2. Preparation of orlistat-rosuvastatin co-crystal complex
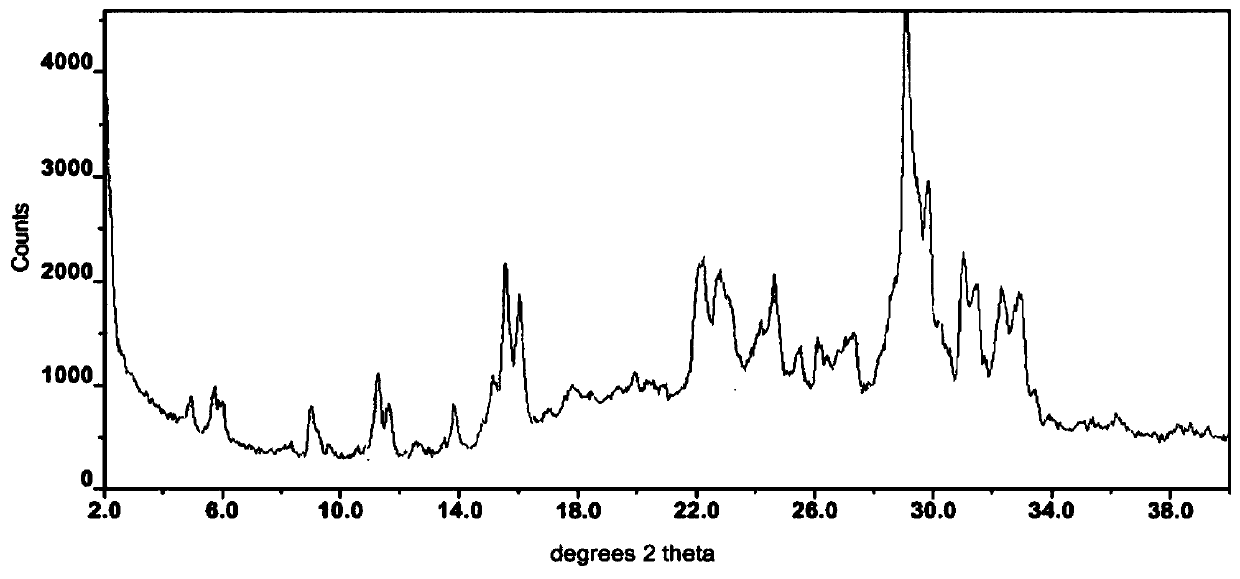
[0073] Take 132.195g of orlistat and 64.205g of rosuvastatin, mix them thoroughly, place them in a planetary ball mill, grind them for 35min at a speed of 350r / min, and collect the product to obtain 178.411g of off-white powder with a melting point of 160.0-162.0°C . Obtain 177.975g of white crystalline powder after recrystallization with acetone, melting point is 162.9~163.9 ℃, R value is 1.932, and its X-ray powder diffraction figure is as follows figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com