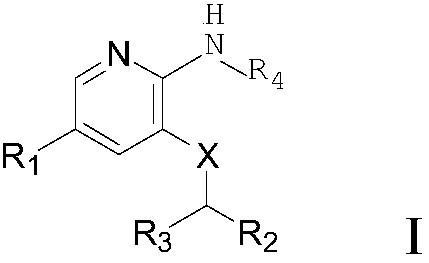
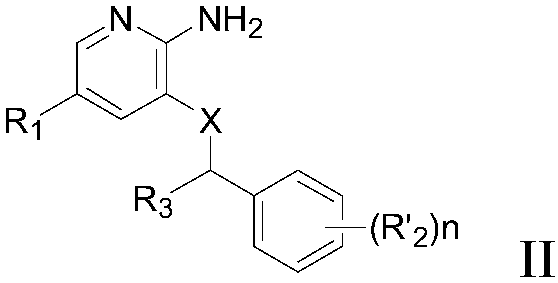
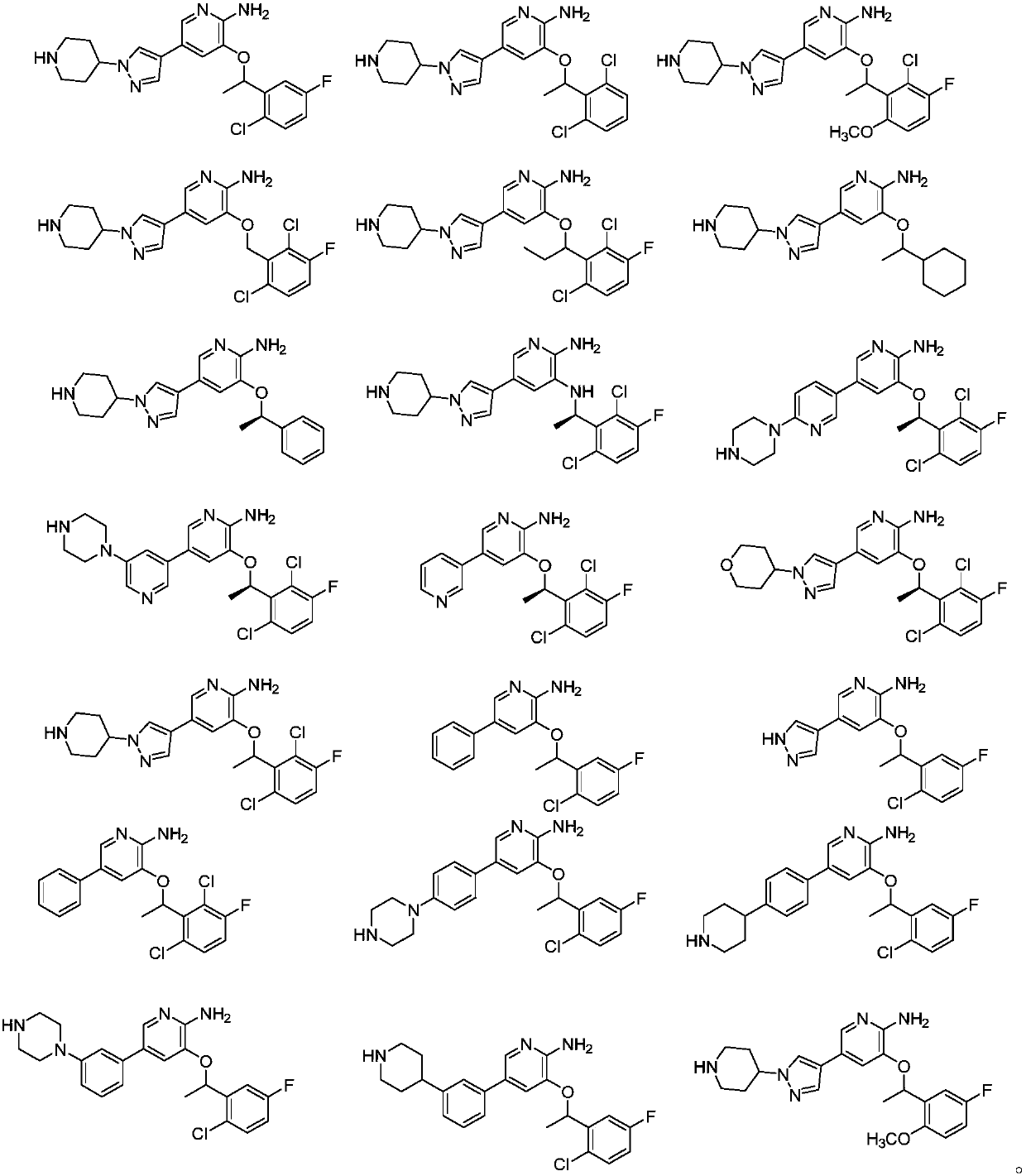
Selective Janus kinase 2 (JAK2) inhibitor and application thereof
A technology selected from, solvate, applied in the field of JAK2 inhibitors and its applications, can solve the problems of side effects, multiple target effects, and low selectivity of inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1 prepares compound WWQ-131
[0085] 1) Synthesis of 1-(2-chloro-5-fluorophenyl)ethanol
[0086]
[0087] Put the raw material (1g, 5.8mmol) in a 50mL reaction flask, dissolve in anhydrous methanol, protect with argon, stir in an ice bath for 30 minutes, then slowly add NaBH 4 (331mg, 8.7mmol), gradually raised to room temperature, stirred at room temperature, TLC tracked the spot plate, and the reaction was completed in 6 hours, quenched with water in an ice bath, spin off methanol, extracted three times with DCM and water, and used for the organic phase Saturated saline was extracted three times, the organic phase was collected, dried with anhydrous sodium sulfate, filtered with suction, and the organic phase was spin-dried to obtain a colorless oily liquid, 960 mg, with a yield of 95%. 1 H NMR (400MHz, CDCl 3 ):δ7.25(dd,J 1 =2.4Hz,J 2 =6.8Hz,1H),7.20(dd,J 1 =4.0Hz,J 2 =5.2Hz,1H),6.83(td,J 1 =2.8Hz,J 2 =8.4Hz, 1H), 5.15(q, J=6.4Hz, 1H), 2.06(s, 1...
Embodiment 2
[0103] Embodiment 2 prepares WWQ-133
[0104] 1) 3-[1-(2,6-dichlorophenyl)ethoxy]-2-nitropyridine
[0105]
[0106] Weigh raw materials 1-(2,6-dichlorophenyl)ethanol (1g, 5.23mmol), 3-hydroxy-2-nitropyridine (807mg, 5.75mmol), triphenylphosphine (1.647g, 6.27mmol) In a 50mL two-necked bottle, add THF to dissolve it. Under argon protection, DIAD (1.236mL, 6.27mmol) was slowly added dropwise under stirring in an ice bath. 50:1), 804 mg of white solid was obtained, and the yield was 48.5%. 1 H NMR (400MHz, CDCl 3 ):δ8.01(dd,J 1 =1.2Hz,J 2 =3.2Hz,1H),7.36-7.33(m,1H),7.32(s,1H),7.30(s,1H),7.23(dd,J=0.8,7.6Hz,1H),7.18(t,J= 8.0Hz, 1H), 6.13(q, J=6.4Hz, 1H), 1.85(d, J=6.8Hz, 3H). GC-MS: m / z=312.1.
[0107] 2) 3-[1-(2,6-dichlorophenyl)ethoxy]-2-aminopyridine
[0108]
[0109] Weigh 3-[1-(2,6-dichlorophenyl)ethoxy]-2-nitropyridine (804mg, 2.56mmol), iron powder (360mg, 6.41mmol) into a 50mL two-necked bottle, add It was dissolved with EtOH, refluxed at 90°C for 30 minute...
Embodiment 3
[0119] Embodiment 3 prepares WWQ-153
[0120] 4-{3-[1-(2,6-dichloro-3-fluorophenyl)methoxy]-2-aminopyridine}pyrazole-1-piperidine
[0121]
[0122] 43mg of white solid was obtained, the yield was 45.1%, melting point: 195.0-196.5°C. 1 H NMR (400MHz, CDCl 3 ): δ7.87(d,J=2.0Hz,1H),7.67(s,1H),7.60(s,1H),7.52(dd,J 1 =2.8Hz,J 2 =6.4Hz,1H),7.39(dd,J 1 =2.4Hz,J 2 =5.6Hz,1H),7.07(d,J=1.6Hz,1H),5.15(s,2H),4.69(s,2H),4.28-4.21(m,1H),3.26(d,J=12.8Hz ,2H),2.78(td,J 1 =2.0Hz,J 2 =12.4Hz,2H),2.19(dd,J 1 =2.0Hz,J 2 =12.4Hz, 2H), 1.98-1.88(m, 2H), 1.83(s, 1H). 13 C NMR (100MHz, CDCl 3 ): δ153.4, 148.6, 140.8, 136.7, 135.8, 130.2, 129.8, 127.7, 126.4, 126.2, 122.9, 122.4, 119.7, 115.2, 63.5, 59.9, 45.7, 34.0. HRMS (ESI) (m / z): [M +H] + calcd for C 20 h 20 Cl 2 FN 5 O, 436.1062; found, 436.1100. HPLC purity: 97.76%, retention time = 10.879min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com