Catalyst for producing ethyl alcohol through C2 acid ester hydrogenation, and preparation and application of catalyst
A catalyst and ethanol production technology, which is applied in the direction of catalyst activation/preparation, hydroxyl compound preparation, metal/metal oxide/metal hydroxide catalyst, etc., which can solve the problems of high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
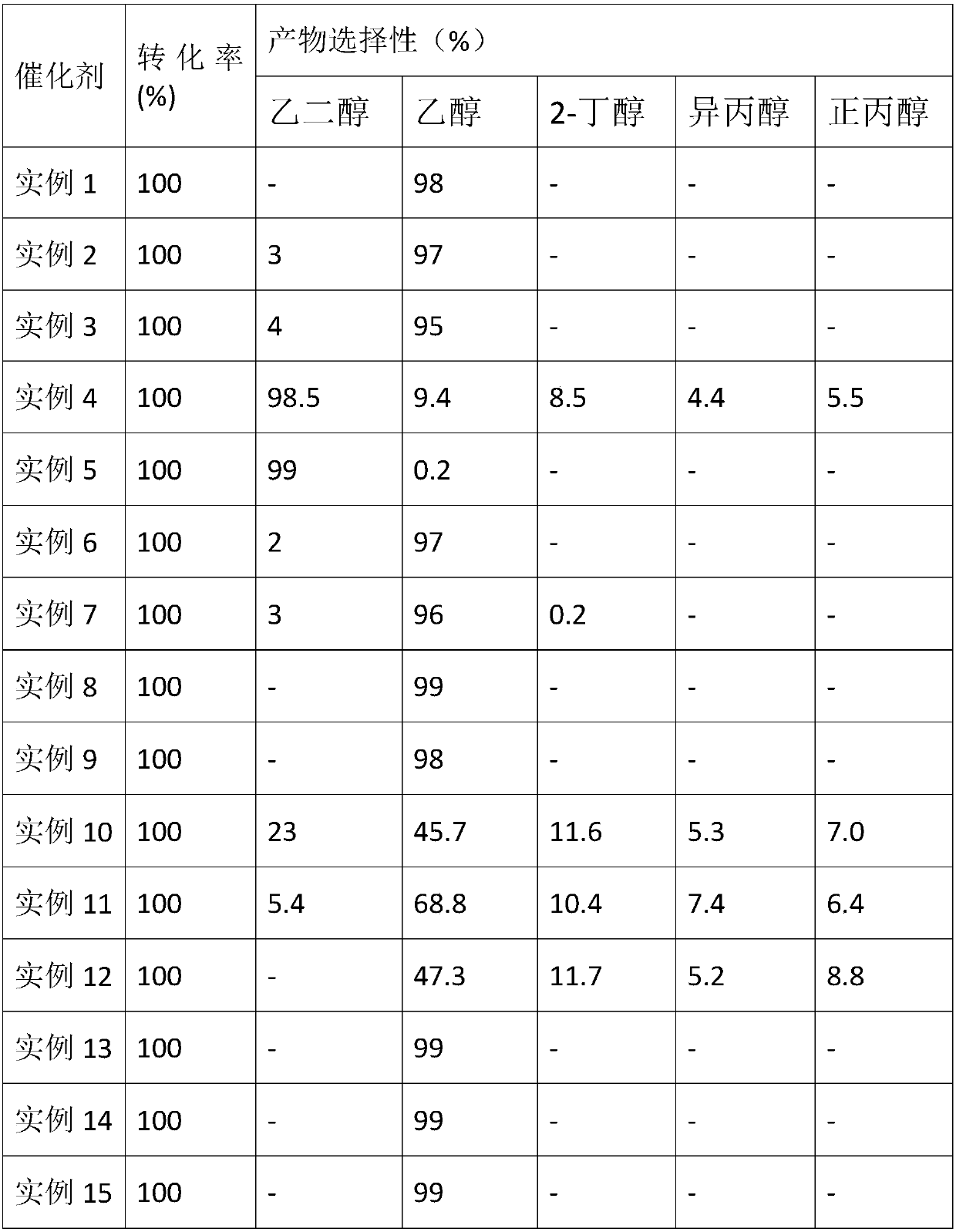
[0021] 0.50g Cu(NO 3 ) 2 ·6H 2 O and 0.56g Co(OAC) 2 ·6H 2 O was dissolved in 100mL deionized water, and then 5.0g mass fraction of 40wt% SiO 2 Sol, adjust the pH value to 11 with ammonia water with a mass fraction of 25%, distill ammonia in a water bath at 80°C for 5 hours, filter, wash, dry, and then calcinate in a muffle furnace at 450°C for 4 hours to obtain 7.6Cu-7.8Co / SiO 2 (where the numbers before the metal represent M 1 Oxide and M 2 The mass percent content of oxide in the catalyst is the same as in the following examples).
[0022] The conditions for the catalytic hydrogenation reaction of dimethyl oxalate are: fixed bed continuous reaction, the reaction raw material is a methanol solution of dimethyl oxalate with a mass concentration of 20%, the hydrogen pressure is 2.5MPa, the reaction temperature is 200°C, and the liquid space velocity is 0.5h -1 , the hydrogen / dimethyl oxalate molar ratio is 200, under the reaction conditions and 7.6Cu-7.8Co / SiO 2 Under ...
Embodiment 2
[0024] 0.86gCu(NO 3 ) 2 ·6H 2 O and 0.16gCo(OAC) 2 ·6H 2 O was dissolved in 100mL deionized water, and then 5.0g mass fraction of 40wt% SiO 2 Sol, adjust the pH value to 11 with ammonia water with a mass fraction of 25%, distill ammonia in a water bath at 80°C for 5 hours, filter, wash, dry, and then calcinate in a muffle furnace at 450°C for 4 hours to obtain 12.1Cu-2.6Co / SiO 2 .
[0025] The conditions for the catalytic hydrogenation reaction of dimethyl oxalate are: fixed bed continuous reaction, the reaction raw material is a methanol solution of dimethyl oxalate with a mass concentration of 20%, the hydrogen pressure is 2.5MPa, the reaction temperature is 200°C, and the liquid space velocity is 0.5h -1 , the hydrogen / dimethyl oxalate molar ratio is 200, under the reaction conditions and 12.1Cu-2.6Co / SiO 2 Under the action of the ethanol yield can reach 97%.
Embodiment 3
[0027] 0.17gCu(NO 3 ) 2 ·6H 2 O and 0.93gCo(OAC) 2 ·6H 2 O was dissolved in 100mL deionized water, and then 5.0g mass fraction of 40wt% SiO 2 Sol, adjust the pH value to 11 with ammonia water with a mass fraction of 25%, distill ammonia in a water bath at 80°C for 5 hours, filter, wash, dry, and then calcinate in a muffle furnace at 450°C for 4 hours to obtain 2.4Cu-13.0Co / SiO 2 .
[0028] The conditions for the catalytic hydrogenation reaction of dimethyl oxalate are: fixed bed continuous reaction, the reaction raw material is a methanol solution of dimethyl oxalate with a mass concentration of 20%, the hydrogen pressure is 2.5MPa, the reaction temperature is 200°C, and the liquid space velocity is 0.5h -1 , the hydrogen / dimethyl oxalate molar ratio is 200, under the reaction conditions and 2.4Cu-13.0 / SiO 2 Under the action of the ethanol yield can reach 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com