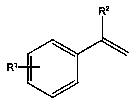
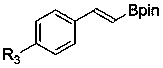
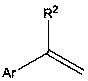
Method for synthesizing alkenyl borate compound through transfer boronation
A technology of alkenyl boronic acid esters and compounds, applied in the fields of organic synthesis and metal catalysis, can solve the problems of borane sensitivity to water and oxygen, difficulty in obtaining substrates, poor compatibility of chemoselective functional groups, etc., and achieve high reaction efficiency and easy operation , the effect of good functional group compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 A method for synthesizing alkenyl boronate compounds by transfer borylation reaction:
[0024]
[0025] Add p-methylstyrene 1a (0.2 mmol, 25 μL), styrene borate pinacol ester 2a (0.4 mmol, 92 mg), toluene (1 mL), Cp 2 ZrH 2 (0.01 mmol, 2.3 mg), stirred reaction at 130 ℃ under nitrogen (1 atm) atmosphere. GC detection until the completion of the reaction 18h. Diatomaceous earth filtration, the solvent was spin-dried under reduced pressure, and the residue was purified by silica gel column chromatography, using petroleum ether: ethyl acetate (100 mL: 1 mL) as the eluent to obtain p-methylstyrene as a colorless oil Pinacol borate 3a (39 mg, 80%).
[0026] The characterization data of this compound are as follows: 1 H NMR (400 MHz, Chloroform- d ) δ 7.40 – 7.36(m, 3H), 7.15 – 7.14 (m, 2H), 6.12 (d, J = 18.4 Hz, 1H), 2.35 (s, 3H), 1.32(s, 12H). 13 C NMR (101 MHz, Chloroform- d ) δ 149.5, 138.9, 134.8, 129.3, 127.0, 83.2, 24.8, 21.3. 11 B NMR (128 MHz, ...
Embodiment 2
[0027] Example 2 A method for synthesizing alkenyl boronate compounds by transfer borylation reaction:
[0028]
[0029] Add p-methylstyrene 1a (0.2 mmol, 25 μL), styrene borate pinacol ester 2a (0.4 mmol, 92 mg), toluene (1 mL), Cp 2 ZrH 2 (0.01 mmol, 2.3 mg), stirred reaction at 130 ℃ under argon (1 atm) atmosphere. GC detection until the completion of the reaction 18h. Diatomaceous earth filtration, the solvent was spin-dried under reduced pressure, and the residue was purified by silica gel column chromatography, using petroleum ether: ethyl acetate (100 mL: 1 mL) as the eluent to obtain p-methylstyrene as a colorless oil Pinacol borate 3a (40 mg, 82%).
[0030] The characterization data of this compound are as follows: 1 H NMR (400 MHz, Chloroform- d ) δ 7.40 – 7.36(m, 3H), 7.15 – 7.14 (m, 2H), 6.12 (d, J = 18.4 Hz, 1H), 2.35 (s, 3H), 1.32(s, 12H). 13 C NMR (101 MHz, Chloroform- d ) δ 149.5, 138.9, 134.8, 129.3, 127.0, 83.2, 24.8, 21.3. 11 B NMR (128 MHz, Chl...
Embodiment 3
[0031] Example 3 A method for synthesizing alkenyl boronate compounds by transfer borylation reaction:
[0032]
[0033] Add p-methylstyrene 1a (0.2 mmol, 25 μL), styrene borate pinacol ester 2a (0.4 mmol, 92 mg), toluene (1 mL), Cp 2 ZrH 2 (0.01 mmol, 2.3 mg), stirred reaction at 60 ℃ under nitrogen (1 atm) atmosphere. GC detection until the completion of the reaction 36h. Diatomaceous earth filtration, the solvent was spin-dried under reduced pressure, and the residue was purified by silica gel column chromatography, using petroleum ether: ethyl acetate (100 mL: 1 mL) as the eluent to obtain p-methylstyrene as a colorless oil Pinacol borate 3a (15 mg, 30%).
[0034] The characterization data of this compound are as follows: 1 H NMR (400 MHz, Chloroform- d ) δ 7.40 – 7.36(m, 3H), 7.15 – 7.14 (m, 2H), 6.12 (d, J = 18.4 Hz, 1H), 2.35 (s, 3H), 1.32(s, 12H). 13 C NMR (101 MHz, Chloroform- d ) δ 149.5, 138.9, 134.8, 129.3, 127.0, 83.2, 24.8, 21.3. 11 B NMR (128 MHz, C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com