2-(3-aminophenoxy)-6-(4-aminophenoxy)-N-(2-aminofluorene)benzamide monomer
A technology of aminophenoxy and benzamide, which is applied in the field of fluorescent active 2--6--N-benzamide monomer and its preparation, can solve the problems of few public reports of polymer monomers, and achieve high fluorescence emission efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of 2,6-difluoro-N-[2-(fluorenylamino)]benzamide:
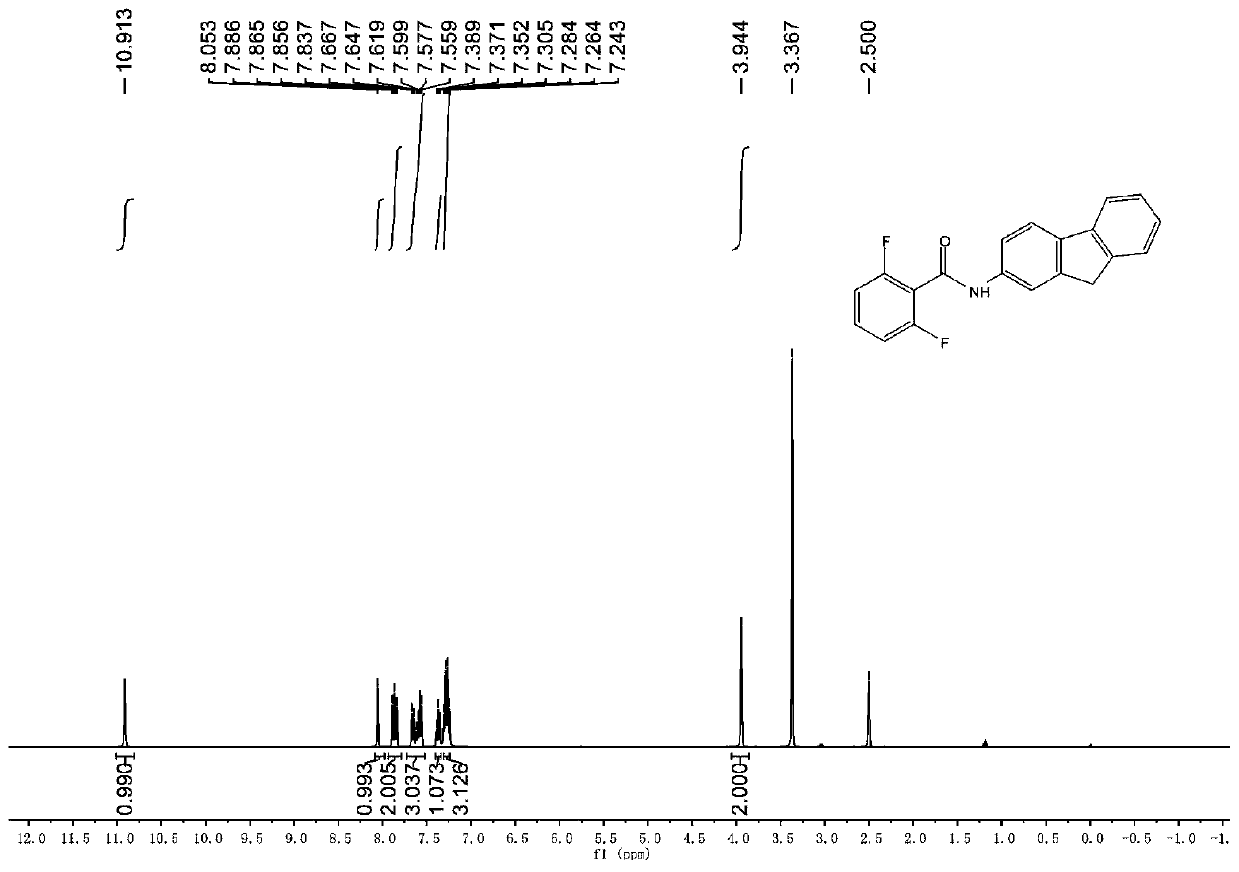
[0041] Under a nitrogen atmosphere and an ice-water bath, 9.06 g (50 mmol) of 2-aminofluorene, 150 mL of dichloromethane and 10.6 mL (75 mmol) of triethylamine were sequentially added to a thick-walled eggplant-shaped bottle (250 mL) equipped with a magnet, After the mixture was stirred well and mixed uniformly, 6.9 mL (65 mmol) of 2,6-difluorobenzoyl chloride was slowly added dropwise to the reaction mixture. The mixture continued to stir under this condition for 8 hours. After the 2-aminofluorene had completely reacted, the reaction was quenched with 100 mL of saturated ammonium chloride aqueous solution. At this time, a large amount of white solid was precipitated in the mixed system, which was suction filtered after standing for half an hour, and the filter cake was After washing with water and petroleum ether, 2,6-difluoro-N-[2-(fluorenylamino)]benzamide 2 was obtained with a yield of 90%.
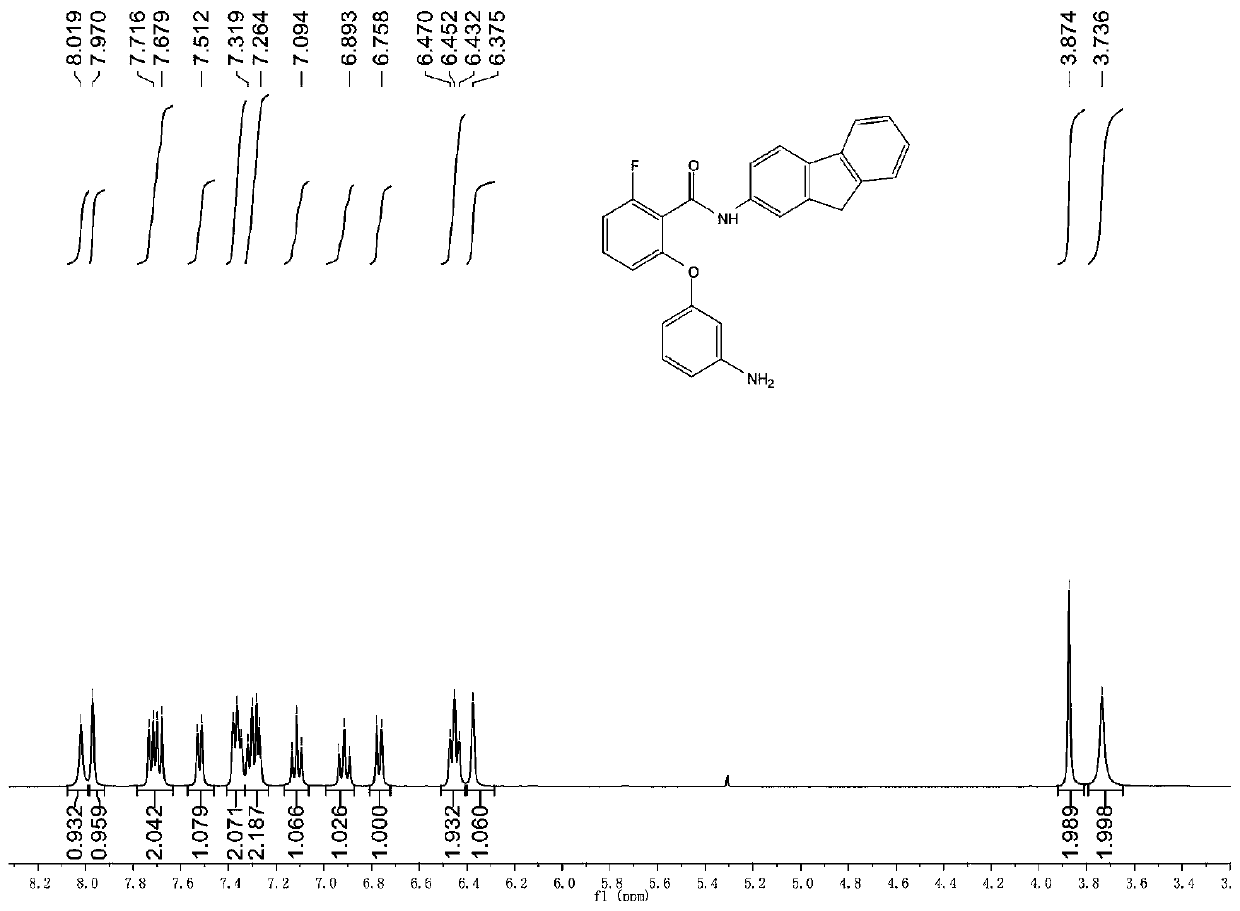
[0042] Synth...
Embodiment 2
[0047] Synthesis of 2,6-difluoro-N-[2-(fluorenylamino)]benzamide:
[0048] Under a nitrogen atmosphere and an ice-water bath, 9.06 g (50 mmol) of 2-aminofluorene, 150 mL of dichloromethane and 10.6 mL (75 mmol) of triethylamine were sequentially added to a thick-walled eggplant-shaped bottle (250 mL) equipped with a magnet, After the mixture was stirred well and mixed uniformly, 6.9 mL (65 mmol) of 2,6-difluorobenzoyl chloride was slowly added dropwise to the reaction mixture. The mixture continued to stir under this condition for 8 hours. After the 2-aminofluorene had completely reacted, the reaction was quenched with 100 mL of saturated ammonium chloride aqueous solution. At this time, a large amount of white solid was precipitated in the mixed system, which was suction filtered after standing for half an hour, and the filter cake was After washing with water and petroleum ether, 2,6-difluoro-N-[2-(fluorenylamino)]benzamide 2 was obtained with a yield of 90%.
[0049] Synth...
Embodiment 3
[0054] Synthesis of 2,6-difluoro-N-[2-(fluorenylamino)]benzamide:
[0055] Under a nitrogen atmosphere and an ice-water bath, 9.06 g (50 mmol) of 2-aminofluorene, 150 mL of dichloromethane and 10.6 mL (75 mmol) of triethylamine were sequentially added to a thick-walled eggplant-shaped bottle (250 mL) equipped with a magnet, After the mixture was stirred well and mixed uniformly, 6.9 mL (65 mmol) of 2,6-difluorobenzoyl chloride was slowly added dropwise to the reaction mixture. The mixture continued to stir for 8 hours under this condition. After the 2-aminofluorene was completely reacted, the reaction was quenched with 100 mL of saturated ammonium chloride aqueous solution. At this time, a large amount of white solid was precipitated in the mixed system, which was suction filtered after standing for half an hour, and the filter cake was After washing with water and petroleum ether, 2,6-difluoro-N-[2-(fluorenylamino)]benzamide 2 was obtained with a yield of 90%.
[0056] Synth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com