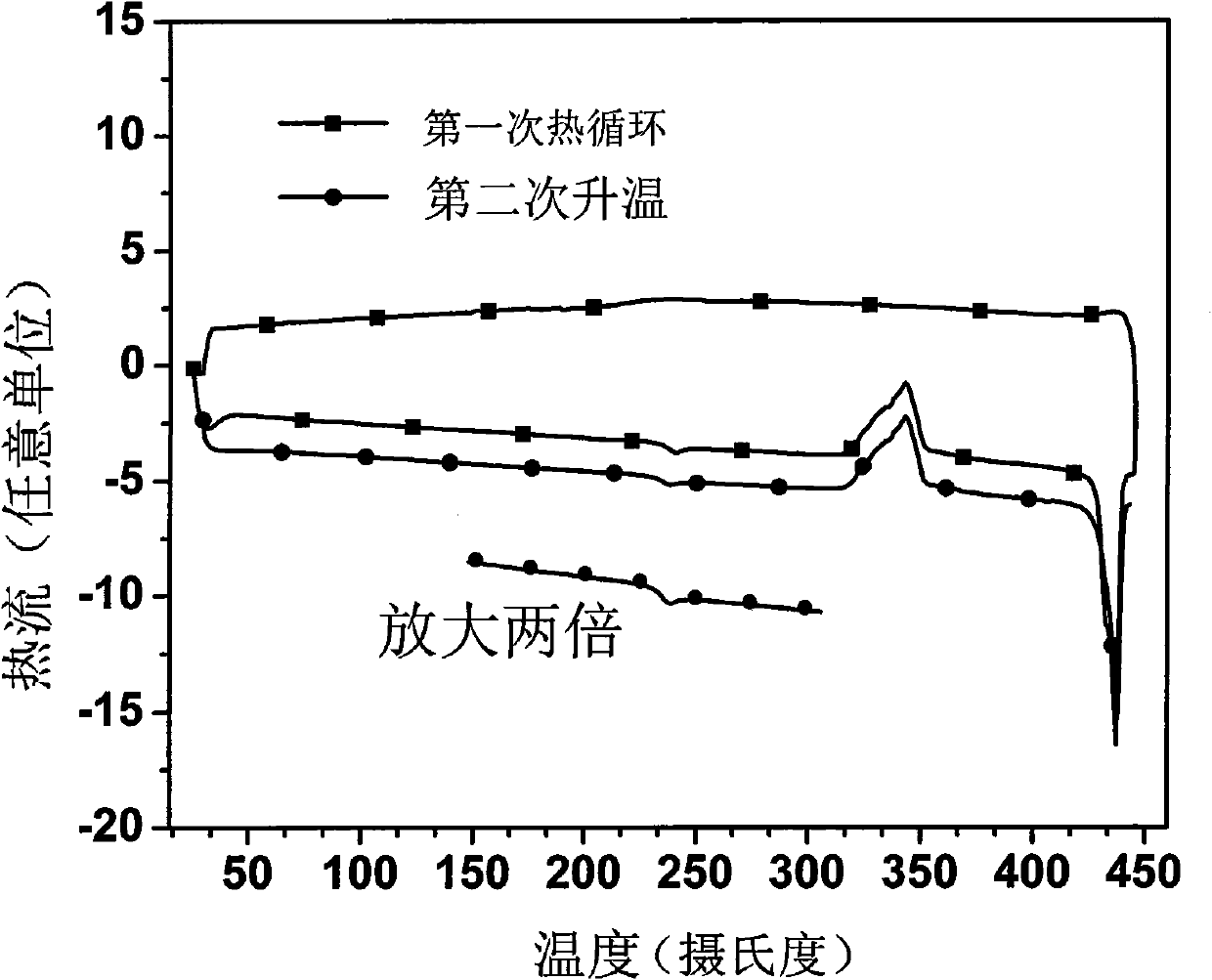
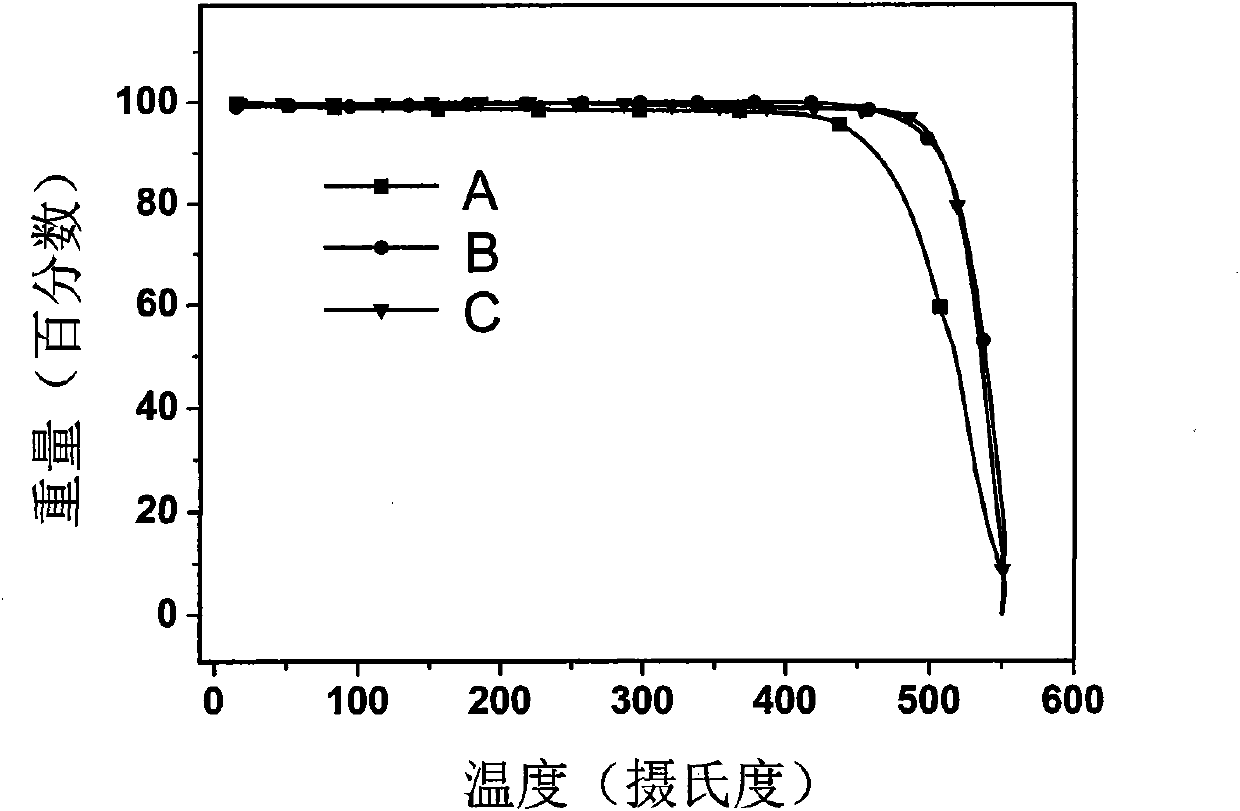
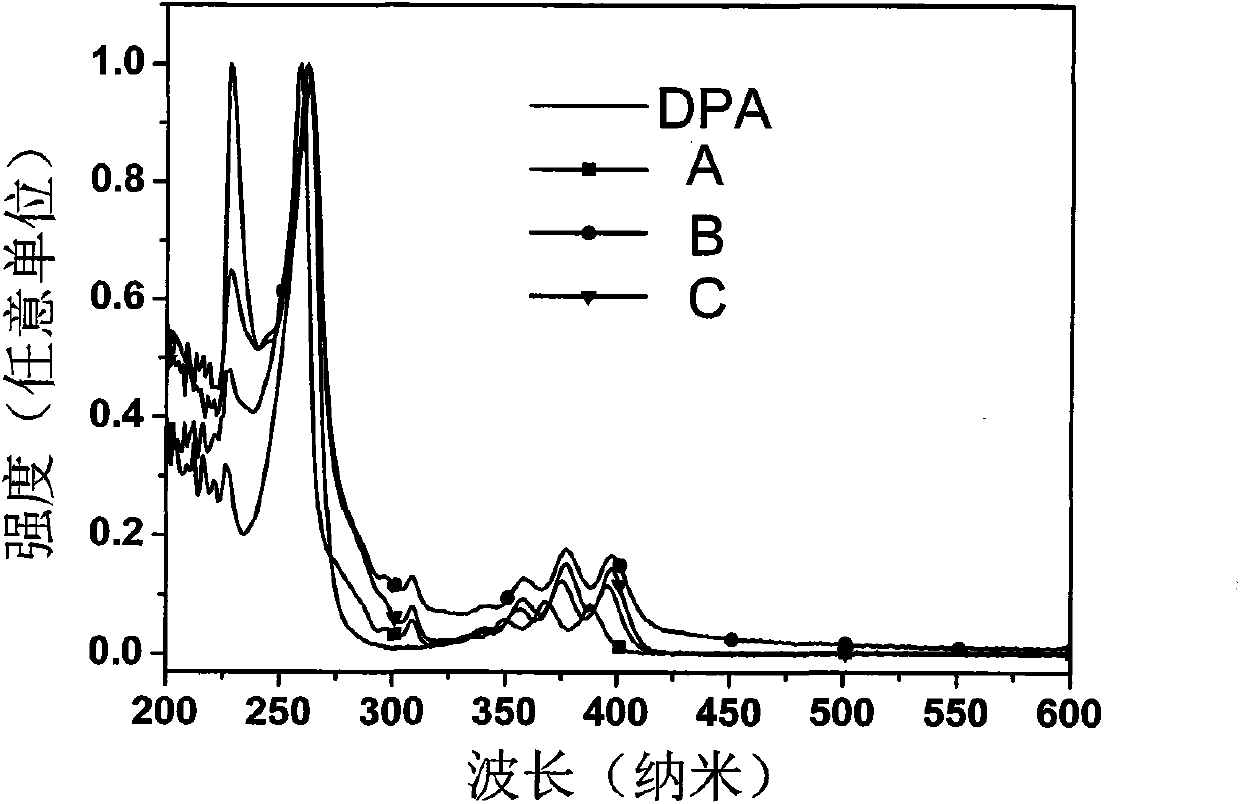
1,3-bis(9-phenylfluorene)-benzanthracene derivative and preparation method thereof
A derivative, the technology of benzanthracene, which is applied in the field of 1,3-bis(9-phenylfluorene)-benzanthracene derivatives and its preparation, can solve the problem of insufficient thermal stability, damage to thin film devices, and luminous efficiency. Problems such as color purity and film-forming performance need to be improved to achieve excellent thermal stability, high luminous efficiency, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Under nitrogen protection, add 15g (0.074mol) of isophthaloyl chloride, 20.7g (0.155mol) of anhydrous aluminum trichloride and 100mL of bromobenzene into a 250ml three-neck flask, stir at room temperature for 8 hours, then heat Continue stirring at 90°C for 2 hours, pour into ice methanol after cooling, and filter to obtain 31.4 g of white 1,3-di-p-bromobenzoylbenzene (compound 1) as a solid, with a yield of 95.3%.
[0029] Identification of compound 1
[0030] Mass Spectrum: [MS(EI)] m / z: C20H12Br2O2 Theoretical 444.1, Actual Measured: 444.
[0031] Proton nuclear magnetic spectrum: 1H-NMR (400MHz, CDCl3) δ (ppm): 8.13 (s, 1H), 8.01-7.99 (d, J=8Hz, 2H), 7.70-7.64 (m, 9H).
[0032] 13C NMR (400MHz, CDCl3, δ): 195.9, 194.8, 138.1, 137.7, 137.5, 137.0, 135.7, 133.9, 133.7, 133.5, 133.1, 132.0, 131.7, 131.2, 131.0, 130.0, 128.9, 128.7, 128.3.
[0033] 2) Under the condition of nitrogen protection, add 3.495g (15mmol) of 2-bromobiphenyl and 50ml of tetrahydrofuran (TH...
Embodiment 2
[0045] 1) with step 1) among the embodiment 1;
[0046] 2) with step 2) in embodiment 1;
[0047] 3) Under nitrogen protection conditions, add 1,3-bis(9-p-bromophenylfluorene)benzene (compound 2) 0.716g (1mmol), 10-phenylanthracene-9-boronic acid pina to a 150ml three-necked flask Esters 0.761g (2mmol), Pd (PPh 3 ) 4 0.035g (0.03mmol), 60ml of toluene and 20ml of 2 moles per liter of potassium carbonate aqueous solution were stirred and refluxed for 24 hours, and the crude product was purified by silica gel chromatography (eluent-petroleum ether / dichloromethane 6: 1v / v) , 0.952 g of white 1,3-bis(9-phenylfluorene)-phenylbiphenylanthracene solid (compound B) was obtained with a yield of 89%.
[0048] Identification of Compound B
[0049] Mass Spectrum: MS (MADLI-TOF): m / z (M+H) + The theoretical value of C84H54 is 1062.42; the actual measured value is 1062.7.
[0050] Proton nuclear magnetic spectrum: 1H NMR (400MHz, CDCl3, δ): 7.848-7.830 (d, J=7.2Hz 4H), 7.788 (s, 1H), ...
Embodiment 3
[0054] 1) with step 1) among the embodiment 1;
[0055] 2) with step 2) in embodiment 1;
[0056] 3) Under nitrogen protection conditions, add 1,3-bis(9-p-bromophenylfluorene)benzene (compound 2) 0.716g (1mmol), 10-naphthyl anthracene-9-boronic acid pinazone into a 150ml three-necked flask Esters 0.861g (2mmol), Pd (PPh 3 ) 4 0.035g (0.03mmol), 60ml of toluene and 20ml of 2 moles per liter of potassium carbonate aqueous solution were stirred and refluxed for 24 hours, and the crude product was purified by silica gel chromatography (eluent-petroleum ether / dichloromethane 6: 1v / v) , 0.911 g of white 1,3-bis(9-phenylfluorene)-benzonaphthylanthracene solid (Compound C) was obtained with a yield of 78%.
[0057] Identification of Compound C
[0058] Mass Spectrum: MS (MADLI-TOF): m / z (M+H) + C92H58, theoretical value 1162.45; actual measured value, 1162.8.
[0059] Proton nuclear magnetic spectrum: 1H NMR (400MHz, CDCl3, δ): 8.057-8.036 (d, J = 8.2Hz, 2H), 8.006-7.985 (d, J =...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com