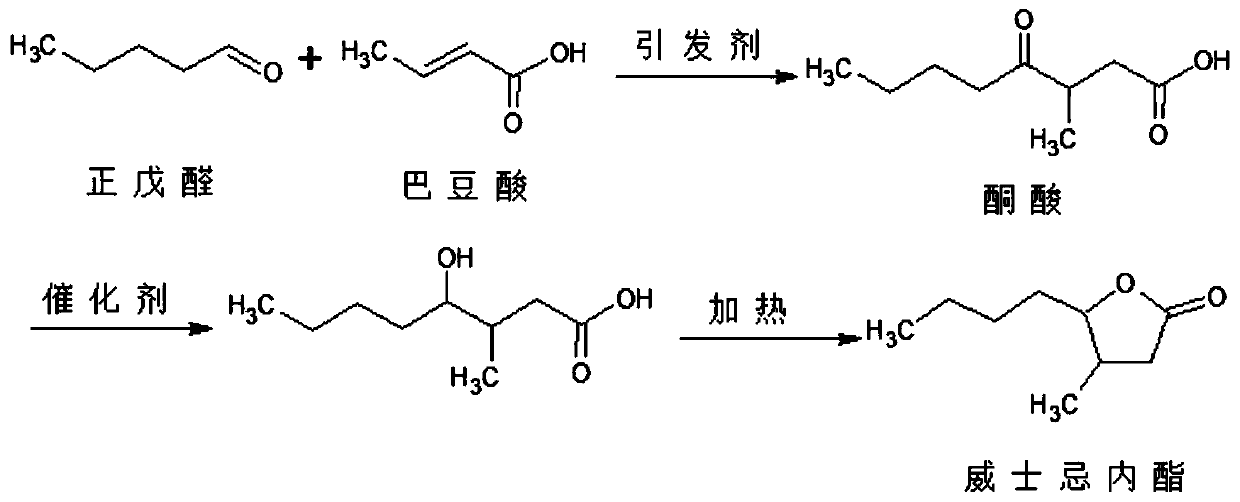
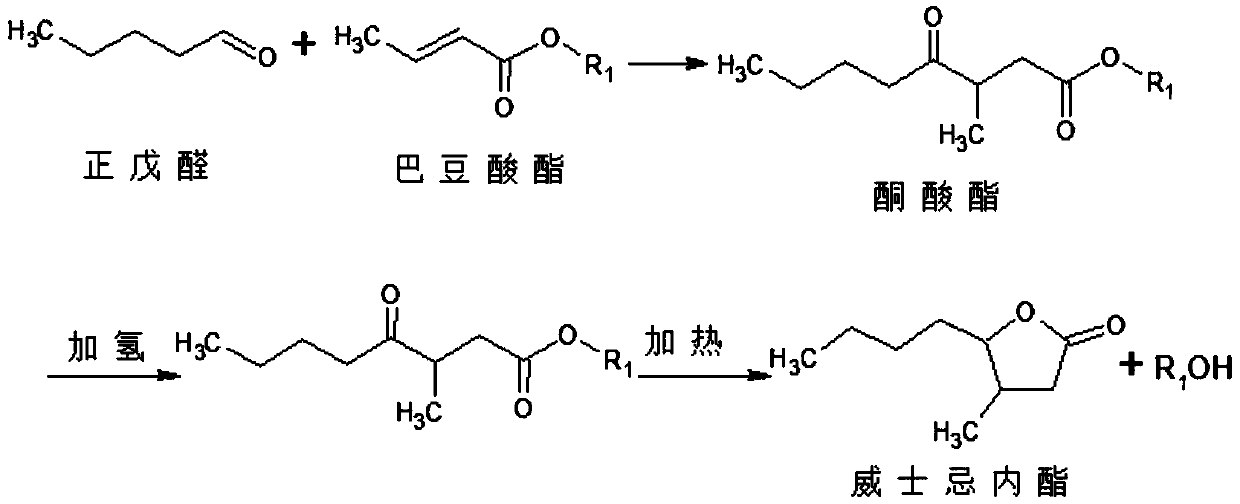
Synthesis process of whisky lactone
A technology of whisky lactone and synthesis process, applied in the direction of organic chemistry and the like, can solve the problems of difficulty in recovery, low conversion rate of n-valeraldehyde, etc., and achieve the effects of improving economic benefits, reducing the difficulty of wastewater treatment, and reducing energy consumption and input cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Synthesis of Ketoesters
[0027] In a 1000mL three-necked flask equipped with a thermometer, a stirrer, and a dropping funnel, add 184g (4mol) of ethanol and 114g (1mol) of ethyl crotonate, mix the above materials evenly, and heat up to reflux, and then pour the mixture through the dropping funnel Add 86 g (1 mol) of n-valeraldehyde (1 mol) and 92 g (2 mol) of ethanol dropwise into a three-necked flask, and stir while dropping. After 2 hours, the dropwise addition is completed, and then continue stirring to react the above reactants at 80° C. for 2 hours, and collect 138 g of distillate. After the reaction finished, the reaction crude product weighed 338g, detected by GC, and recorded that the main fraction was ketoester, and the content was 92%; ethyl crotonate had 6% unreacted (after deducting the solvent ethanol peak).
[0028] 2) Synthesis of whiskey lactone
[0029] In an autoclave with a stirring bar, add 338 g of crude keto ester and 6 g of a Pd / C catalyst wi...
Embodiment 2
[0031] 1) Synthesis of Ketoesters
[0032] In a 1000mL three-necked flask equipped with a thermometer, a stirrer, and a dropping funnel, add 230g (5mol) of ethanol and 114g (1mol) of ethyl crotonate, mix the above materials evenly, and heat up to reflux, and then pour the mixture through the dropping funnel. Add 86 g (1 mol) of n-valeraldehyde and 46 g (1 mol) of ethanol dropwise into a three-necked flask, and stir while dropping. After 2 hours, the dropwise addition is completed, and then continue to stir to react the above reactants at 80° C. for 2 hours, and collect 132 g of distillate. After the reaction finished, the reaction crude product weighed 342g, detected by GC, and recorded that the main fraction was ketoester, and the content was 89.2%; ethyl crotonate had 8% unreacted (after deducting the solvent ethanol peak).
[0033] 2) Synthesis of whiskey lactone
[0034] Add 342g of ketoester crude product and 6g of mass fraction 5% Pd / C catalyst in an autoclave with a st...
Embodiment 3
[0036] 1) Synthesis of Ketoesters
[0037] In a 1000mL three-necked flask equipped with a thermometer, a stirrer, and a dropping funnel, add 300g (5mol) of propanol and 128g (1mol) of propyl crotonate, mix the above materials evenly, heat up to reflux, and then pass through the dropping funnel Add 86g (1mol) of n-valeraldehyde and 60g (1mol) of propanol dropwise to the three-necked flask, and stir while dropping. After 2h, the dropwise addition is completed, and then continue to stir, so that the above reactants are reacted at 102°C for 2h, and the distillate is collected. 180g. After the reaction finished, the reaction crude product weighed 480g, and GC was detected, and the main fraction was recorded as ketoester, and the content was 93%; 4% of propyl crotonate was unreacted (after deducting the peak of solvent propanol).
[0038] 2) Synthesis of whiskey lactone
[0039] In an autoclave with a stirring bar, add 480 g of crude keto ester and 10 g of a 5% Pd / C catalyst by ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com