Preparation method of carbon-supported nano-palladium catalyst for electrochemical oxidation of methanol
A nano-palladium, electrochemical technology, applied in the direction of circuits, electrical components, battery electrodes, etc., can solve the problems of resource scarcity, direct methanol fuel cells that are difficult to commercialize, and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
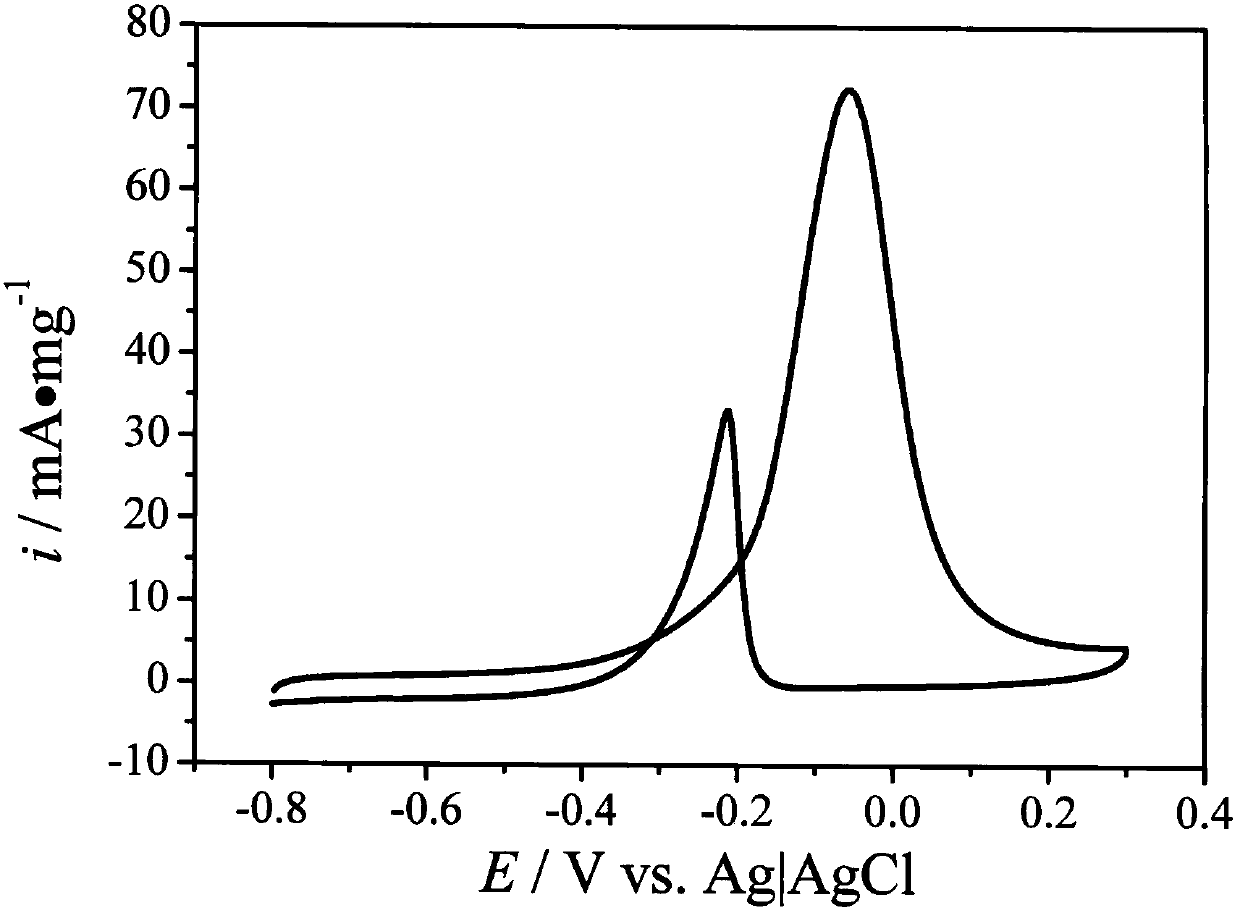
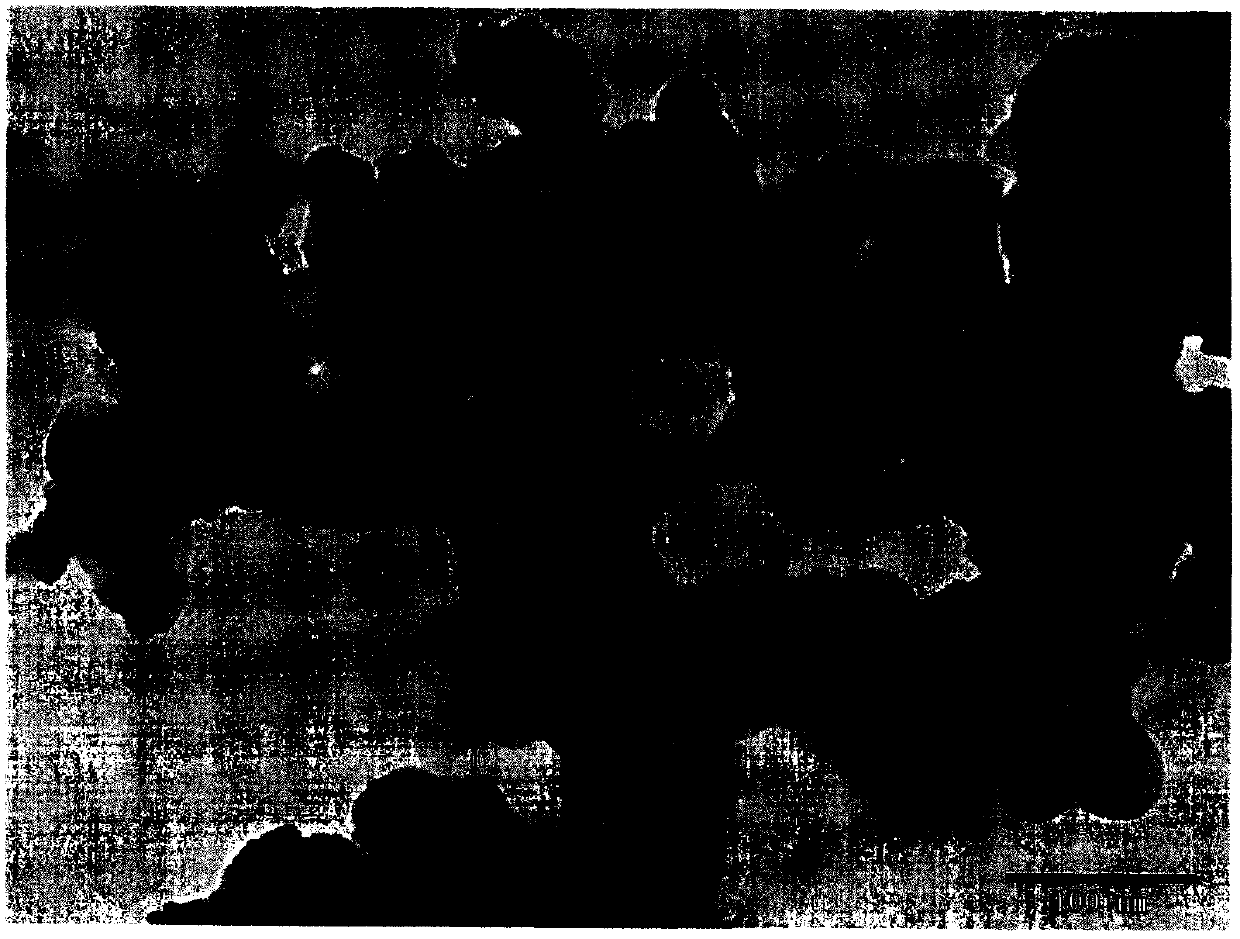
[0021] Embodiment 1: In the third step of the preparation process, the amount of KOH solution is 3.4ml; other preparation conditions remain unchanged. The TEM picture of the obtained catalyst is as figure 1 shown. figure 1 It was shown that palladium nanoparticles were successfully supported on the surface of the activated carbon support. figure 2 is the resulting catalyst in 0.1M KOH+1M CH 3 CV curves in OH mixed solution. The current density of the CV curves was normalized by the mass of the catalyst. From figure 2 It can be seen that the peak electrode potential of the current density of the electrochemical oxidation of methanol on the catalyst surface is -0.05V. At this potential, the current density of the electrochemical oxidation of methanol is 72.6mA·mg -1 catalyst.
Embodiment approach 2
[0022] Embodiment 2: In the third step of the preparation process, the amount of KOH solution is 4.4ml; other preparation conditions remain unchanged. The TEM picture of the obtained catalyst is as image 3 shown. image 3 It was shown that palladium nanoparticles were successfully supported on the surface of the activated carbon support. Figure 4 is the resulting catalyst in 0.1M KOH+1M CH 3 CV curves in OH mixed solution. The current density of the CV curves was normalized by the mass of the catalyst. From Figure 4 It can be seen that the peak electrode potential of the current density of the electrochemical oxidation of methanol on the catalyst surface is -0.06V. At this potential, the current density of the electrochemical oxidation of methanol is 85.02mA·mg -1 catalyst.
Embodiment approach 3
[0023] Embodiment 3: In the third step of the preparation process, the amount of KOH solution is 5.4ml; other preparation conditions remain unchanged. The TEM picture of the obtained catalyst is as Figure 5 shown. Figure 5 It was shown that palladium nanoparticles were successfully supported on the surface of the activated carbon support. Figure 6 is the resulting catalyst in 0.1M KOH+1M CH 3 CV curves in OH mixed solution. The current density of the CV curves was normalized by the mass of the catalyst. From Figure 6 It can be seen that the peak electrode potential of the current density of the electrochemical oxidation of methanol on the catalyst surface is -0.05V. At this potential, the current density of the electrochemical oxidation of methanol is 85.39mA·mg -1 catalyst.
[0024] Embodiment 4: In the third step of the preparation process, the amount of KOH solution is 6.4ml; other preparation conditions remain unchanged. The TEM picture of the obtained catalyst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com