Use of glucocorticoid receptor modulators in the treatment of catecholamine-secreting tumors
A glucocorticoid, receptor modulator technology, applied in the therapy and composition of patients with Cushing's syndrome, the treatment of Cushing's syndrome patients with neuroendocrine tumors, the field of Cushing's syndrome in patients, to achieve improved methods and treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
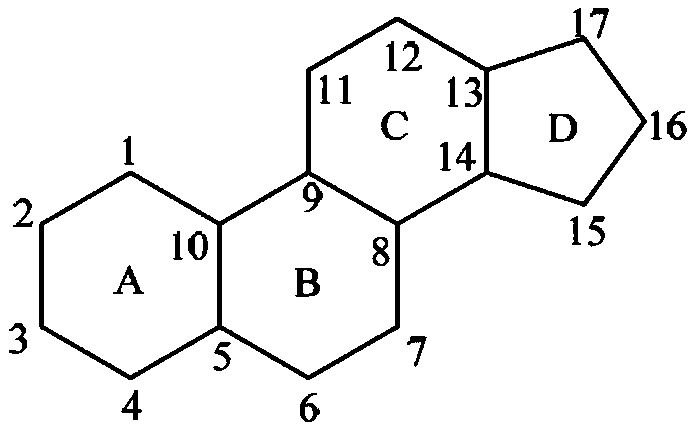
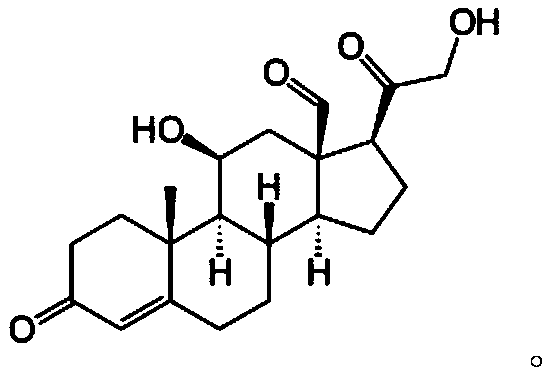
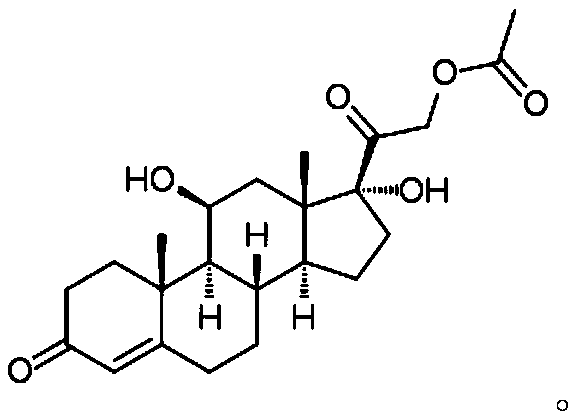
Image
Examples
Embodiment 1
[0194] A phase 2 study of somatostatin analogues in a 53-year-old female patient with metastatic pheochromocytoma associated with ectopic ACTH secretion, response to CVD (cyclophosphamide, vincristine, and dacarbazine ) and sunitinib (tyrosine kinase inhibitor) conventional chemotherapy unresponsive. After 3 months of treatment, despite high doses of atenolol (beta-blocker) and phenoxybenzamine (alpha-blocker), her metanephrine levels were still significantly elevated High and continue to experience significant catecholamine overdose symptoms (palpitations, tremors, and panic attacks). At the same time, a diagnosis of Cushing's syndrome was made, and 300 mg of mifepristone was added to his treatment regimen, which was further increased to 600 mg per day after two weeks. After one week of taking mifepristone, a significant improvement in symptoms of cortisol excess and catecholamine excess was noted. Plasma adrenaline levels were reduced by 50% after 2 weeks of mifepristone t...
Embodiment 2
[0196] Patients with metastatic or unresectable catecholamine-secreting tumors were given an effective amount of mifepristone at a daily dose of 50 mg / kg / day, said patients did not require treatment with glucocorticoid receptor antagonists and were not receiving Treatment with exogenous glucocorticoid receptor agonists. Measurements showed that blood levels of catecholamines were lower after five weeks of treatment compared to initial levels.
Embodiment 3
[0198] Patients with metastatic or unresectable catecholamine-secreting tumors were given an effective amount of mifepristone at a daily dose of 20 mg / kg / day, said patients did not require treatment with glucocorticoid receptor antagonists and were not receiving Treatment with exogenous glucocorticoid receptor agonists. Measurements showed that blood levels of catecholamines were lower after five weeks of treatment compared to initial levels.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com