Test method and device for improving in-vivo and in-vitro correlation of inhaled preparation
A technology for inhaled preparations and testing equipment, which is applied in the field of testing and equipment to improve the in-vivo and in-vitro correlation of inhaled preparations, and can solve problems such as obvious gaps, inability to be simulated by cascade impactors, and inability to represent different groups of people.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment example 1
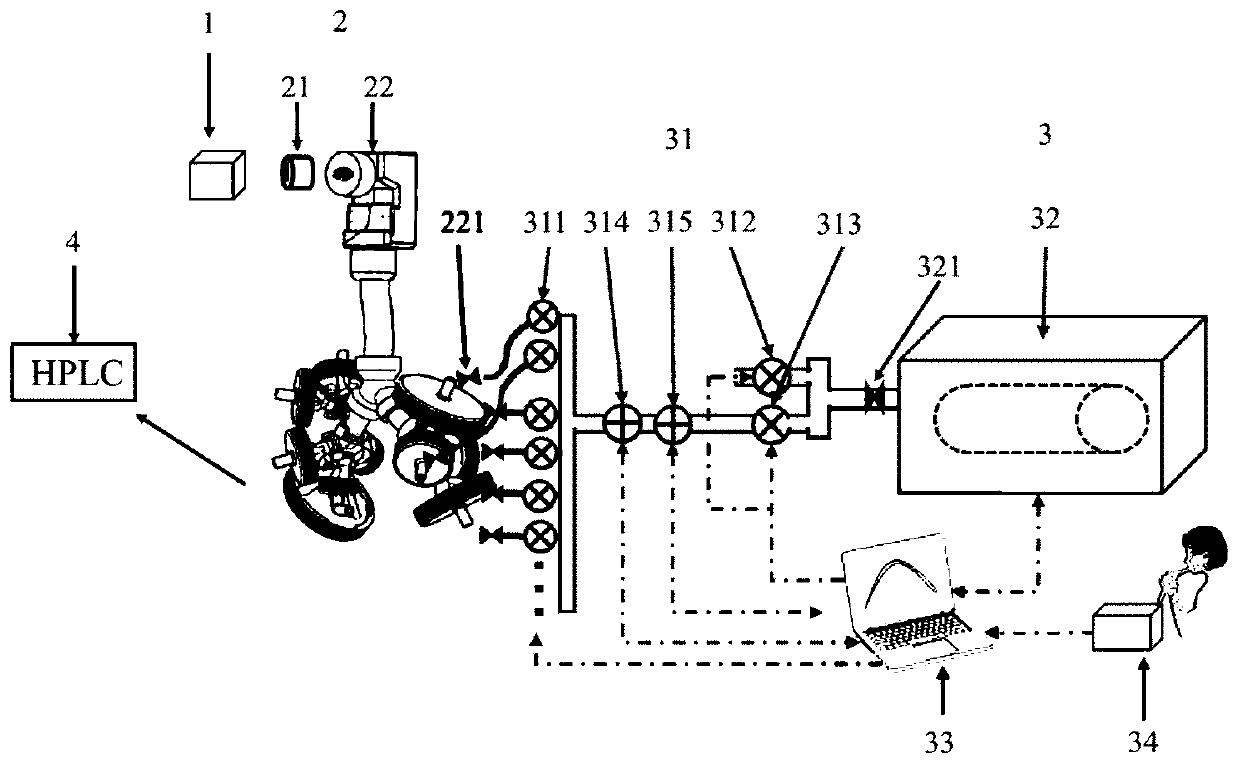
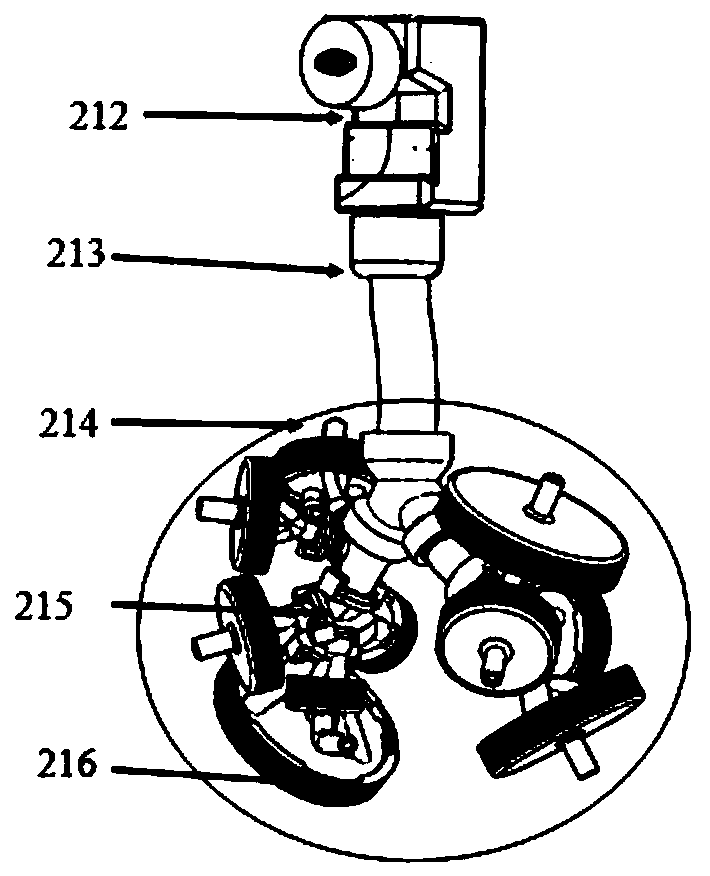
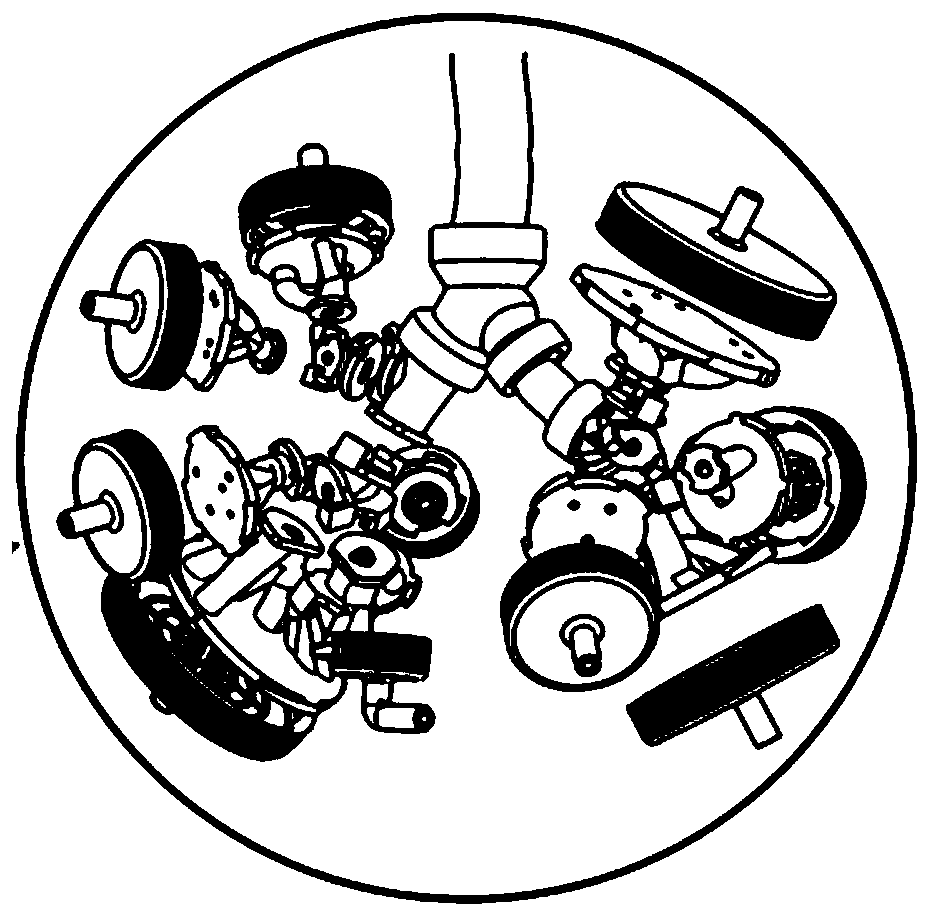
[0039] like figure 1 As shown, it is a schematic structural view of the test equipment for improving inhalation preparation in vivo and in vitro correlation of the present invention; as figure 2 Shown is a schematic structural diagram of a real human respiratory tract 3D solid model. A test device for improving the in vivo and in vitro correlation of inhalation preparations, which includes: a drug delivery device 1, a human respiratory tract model 2, and a breathing simulator 3, wherein the human respiratory tract model 2 includes an adapter 21, a real human respiratory tract 3D solid model 22, and breathing simulation The machine 3 includes a connection control assembly 31, a piston mover 32, a control system 33 and a respiratory data acquisition device 34, and is characterized in that: one end of the adapter 21 is connected with the drug delivery device 1, and the other end is connected with the real human respiratory tract 3D solid model 22, And it can ensure that the joi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com