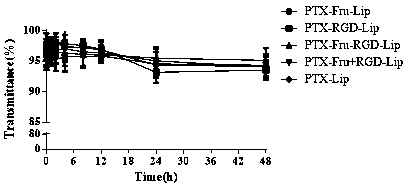
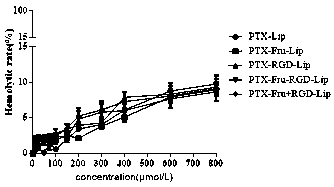
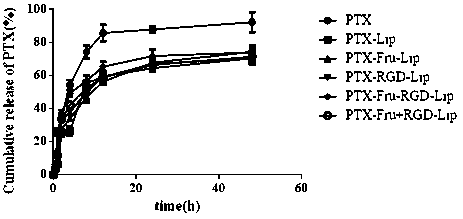
Fructose and RGD peptide co-modified dual-targeting triple-negative breast cancer lipid material
A dual-targeting technology for triple-negative breast cancer, applied in liposome delivery, organic active ingredients, medical preparations of non-active ingredients, etc., can solve problems such as limited targeting ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of compound 2
[0036]
[0037] Under argon protection, fructose-free 2 (2.00 g, 11.10 mmol) was added to a mixed solution of 39 mL of anhydrous acetone and 1.95 mL of sulfuric acid at 0 °C. The reaction solution was moved to room temperature and reacted for 2 h. The completion of the reaction was monitored by TLC, and 0 °C aqueous sodium hydroxide solution (6.10 g, 152.50 mmol, 55 mL) was slowly added to the reaction solution under an ice bath. Suction filtration, distill off the solvent in the filtrate under reduced pressure, dissolve the residue in dichloromethane (50 mL), wash with water (100 mL × 2), saturated brine (100 mL × 2) successively, anhydrous sodium sulfate Dry, filter, remove excess solvent under reduced pressure, recrystallize petroleum ether to obtain 2.10 g of white solid, yield 72.66%. Mp: 94-96°C (Literature Mp: 95-96°C).
Embodiment 2
[0039] Preparation of compound 3
[0040]
[0041] Under argon protection, compound 2 (2.00 g, 7.68 mmol), succinic anhydride (923 mg, 9.23 mmol) and 4-dimethylaminopyridine (DMAP, 94 mg, 0.77 mmol) were dissolved in anhydrous toluene and anhydrous In a mixed solution of triethylamine (30 mL:1 mL), the reaction solution was refluxed in an oil bath at 110 °C for 2.5 h. The completion of the reaction was monitored by TLC, the toluene was distilled off under reduced pressure, the residue was dissolved in a mixed solution of 38 mL of dichloromethane and 54 mL of water, and the pH was adjusted to neutral with acetic acid. The aqueous layer was extracted with dichloromethane (40mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 2.69 g of a colorless oil with a yield of 97.11%. The product was directly carried on to the next step without purification. 1 H-NMR (600 MHz, CDCl 3 , ppm)δ: 1.34...
Embodiment 3
[0043] Preparation of Compound 6
[0044]
[0045] Compound 4 (1.14 g, 6.51 mmol) was dissolved in 30 mL of tetrahydrofuran, N-methylmorpholine (NMM, 0.717 mL, 6.51 mmol) was added, and isobutyl chloroformate (IBCF, 0.823 mL, 6.51 mmol), after the dropwise addition, continue to activate at -10 ℃ for 30 min. Compound 5 (3.16 g, 6.51 mmol) was dissolved in a mixed solution of tetrahydrofuran (10 mL) and N-methylmorpholine (0.717 mL), and added dropwise to the activation solution above. After the dropwise addition was completed, the reaction was carried out at room temperature for 4 h, and the completion of the reaction was monitored by TLC. The solvent in the reaction solution was removed under reduced pressure, and the residue was dissolved in 30 mL of dichloromethane, followed by dilute hydrochloric acid (1 N, 50 mL × 2), saturated sodium bicarbonate solution (50 mL × 2) and saturated sodium chloride solution (50 mL × 2) for washing. The organic phase was dried over anhy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com