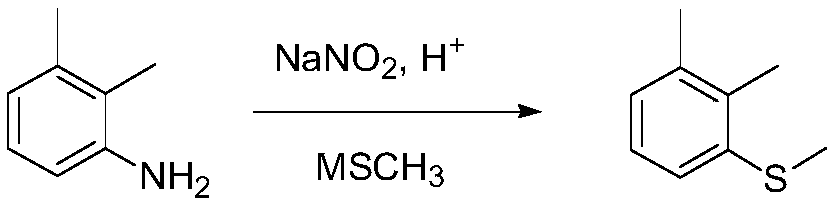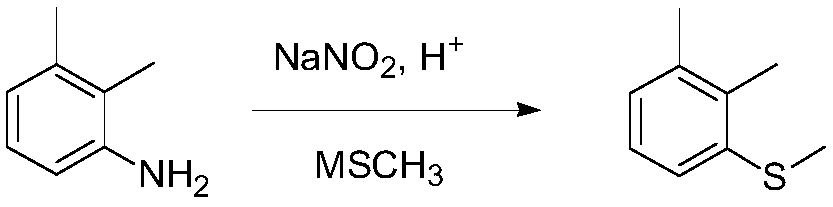Synthetic method of topramezone intermediate 1,2-dimethyl-3-methylsulfanyl-benzene
A kind of technology of dimethyl anisole and oxaflutole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Put 300g of 10%wt hydrochloric acid into a 500mL four-necked bottle; add 50g (0.41mol) of 2,3-dimethylaniline dropwise while stirring, and solids will precipitate during the dropwise addition; heat up to about 50°C and stir after dropping 30 minutes. Cool the reaction solution to -5°C~0°C, add dropwise sodium nitrite aqueous solution (29g sodium nitrite dissolved in 50g water) (0.42mol), and control the dropwise reaction temperature between -5°C~0°C. After the dropwise addition, keep stirring at -5°C to 0°C for 1 hour to obtain a diazonium salt solution.
[0025] (2) Add 433.2g (1.24mol) of 20% sodium methyl mercaptide aqueous solution to another 1L four-necked bottle, control it not to exceed 35°C, add the above-mentioned diazonium salt solution dropwise, and drop it for about 1 hour; continue to keep warm and stir after dropping Reaction 1h.
[0026] (3) Post-treatment: add dropwise 30% hydrochloric acid to adjust the pH of the reaction solution to about 6, let ...
Embodiment 2
[0028] (1) Put 200g of 20%wt sulfuric acid into a 1L four-necked bottle; add 50g (0.41mol) of 2,3-dimethylaniline dropwise while stirring, and solids will precipitate during the dropping process; heat up to about 50°C and stir after dropping 30 minutes. Cool the reaction solution to -10°C ~ -5°C, add dropwise sodium nitrite aqueous solution (56.6g sodium nitrite dissolved in 100g water) (0.82mol), control the dropwise reaction temperature between -10°C ~ -5°C ; After the dropwise addition, keep stirring at -10°C to -5°C for 1 hour to obtain a diazonium salt solution.
[0029] (2) Add 881.5 g (2.05 mol) of 20% potassium methyl mercaptide aqueous solution to another 2L four-necked bottle, control the reaction temperature at 20-35°C, add the above-mentioned diazonium salt solution dropwise, and finish dropping in about 2 hours; Continue to insulate and stir the reaction for 2h.
[0030] (3) Post-treatment: add dropwise 30% hydrochloric acid to adjust the pH of the reaction solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com